Lentivirus Tools
Lentiviruses are a type of retrovirus that can infect both dividing and non-dividing cells, making them an attractive vector for gene therapy applications. Using lentiviral vectors, therapeutic genes can be delivered into patient cells in order to stably replace, modify or supplement defective or missing genes from a patient's genome. Lentiviral vectors are also commonly used to deliver and express chimeric antigen receptor (CAR) in T cells and other immune cells.
Our lentiviral vector product offerings include a novel p24 immunoassay, in-process potency testing tools for T cell redirecting lentivirus and a broad portfolio of genomics solutions for lentivirus characterization and analysis.
Lumit® p24 Immunoassay
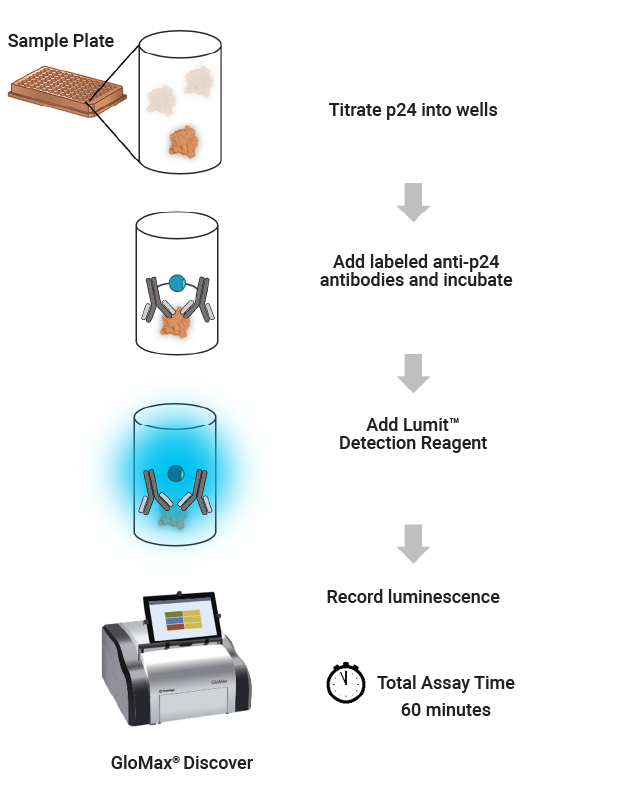
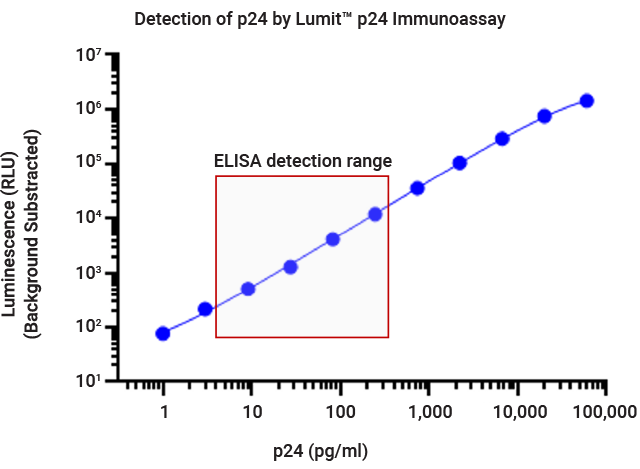
In a gene therapy workflow, reproducible detection of the p24 protein on lentivirus capsids is an essential measurement in determining lentiviral titer. Using our Lumit® technology, we have developed an easy-to-use immunoassay that provides rapid detection of the p24 protein.
The Lumit® p24 Immunoassay offers a simple, no-wash protocol, broad dynamic range and can be completed in as little as 30 minutes, making it a compelling alternative to conventional labor-intensive immunoassays such as ELISAs.
Contact us for more information on custom Lumit® antibody labeling or assay development.
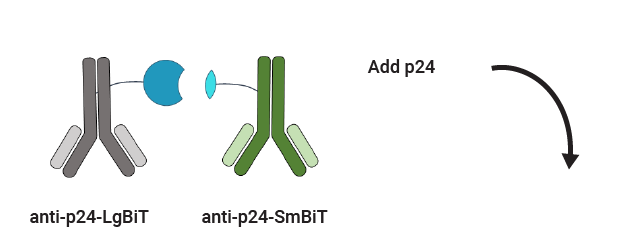
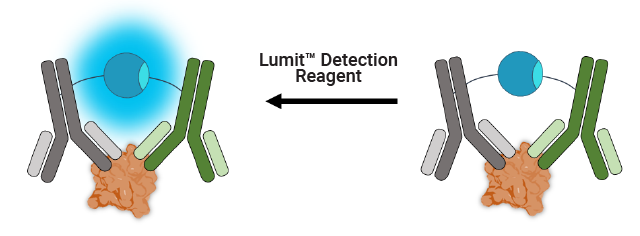
Lumit™ p24 Immunoassay Workflow
Interested in the Lumit® p24 Immunoassay?
In-Process Potency Testing of T Cell Redirecting Lenti-CAR Virus
For lentivirus vectors used in CAR-T manufacturing, guidance from regulatory authorities recommends additional measures of potency beyond transgene expression of the viral vector in CAR-T cells.
To address this need, we developed a MoA-based potency assay to measure CAR:target cell engagement for T cell redirecting lentiviral vectors. The bioassay provides a stability indicating, quantitative luminescent readout that is readily implemented as part of your CAR-T workflow.
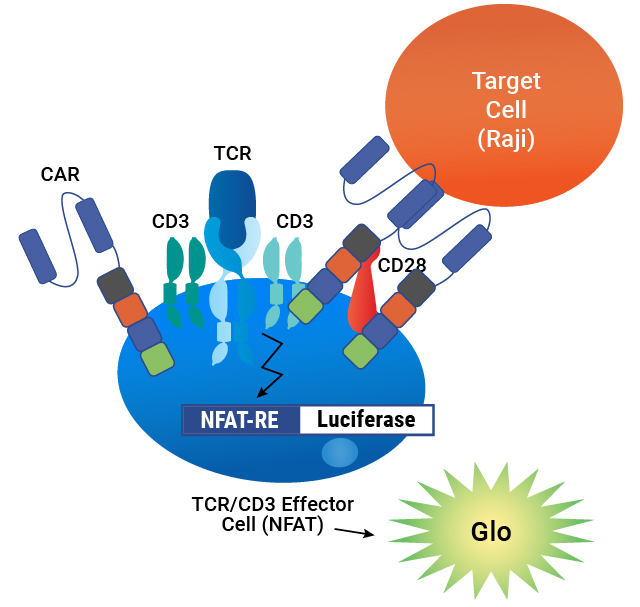
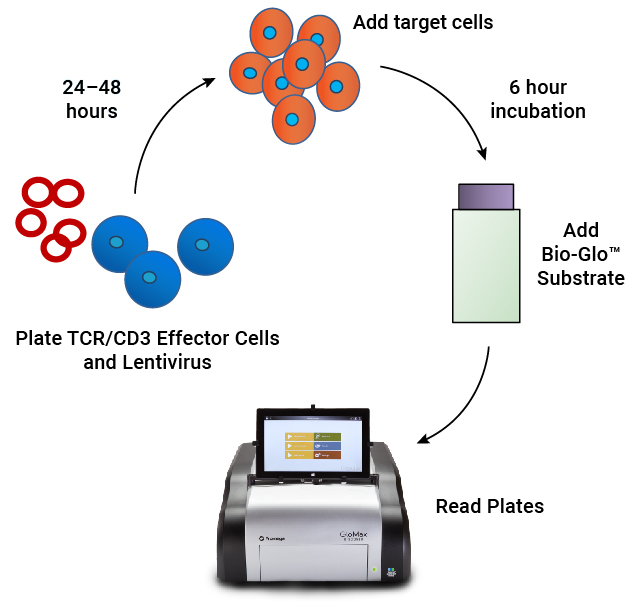
Lentivirus Bioassay is Specific


Lentivirus Bioassay is Stability Indicating

| Heat Treatment Time (hours) |
EC50 |
% Inhibition of Max |
|---|---|---|
| 0 |
0.81 | Reference |
| 2 |
1.0 |
26% |
| 6 |
2.4 |
18% |
| 24 | 2.5 |
47% |
| 48 | 5.0 |
67% |
Forced degradation samples of CAR-19 lentivirus were prepared by incubating at 37°C for 2–48 hours, then analyzed using TCR/CD3 Effector Cells and Raji Target Cells. The assay demonstrates a loss of lentiviral potency with heat treatment, as measured by an increase in EC50 and a decrease in the maximum response.
Automated RNA or DNA Extraction for Lentiviral Vector Characterization
The Maxwell RSC® Instrument is magnetic particle mover that uses prefilled cartridges and pre-programmed methods to extract DNA and/or RNA from a wide variety of sample types in 25–60 minutes.
We also have high-throughput extraction chemistries and manual extraction solutions for RNA and DNA if you are working on a smaller scale.

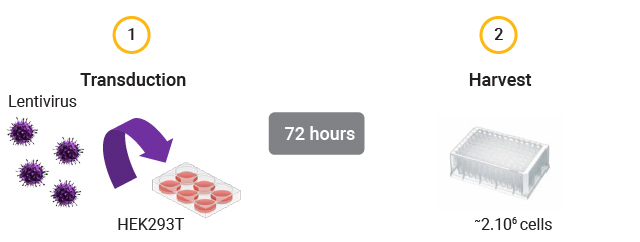
Workflow of gDNA Extraction for Infectious Titer Determination After Cell Transduction
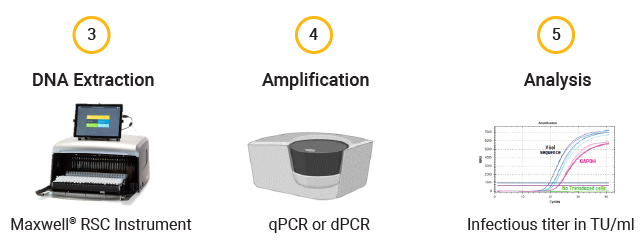
Workflow of RNA Extraction for Copy Number Determination of Produced Lentiviral Vector Batches

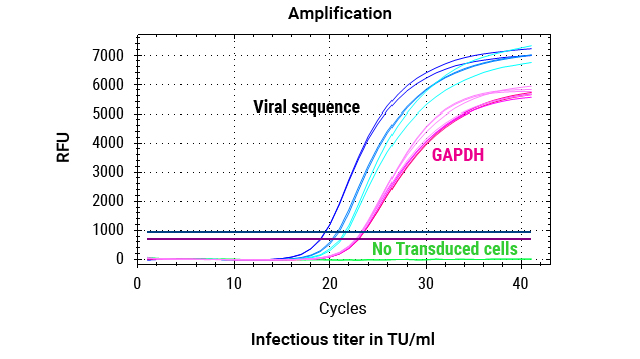
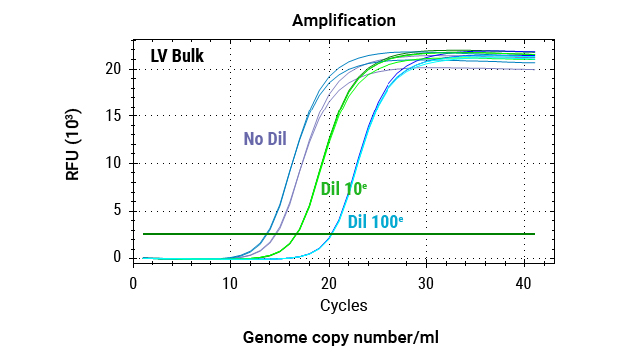
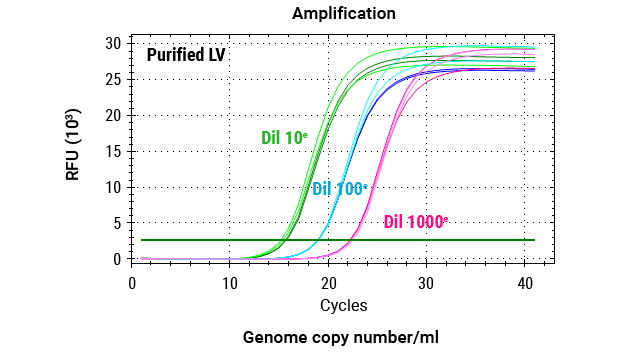
DNA was extracted from 2 million cells transduced with 3 dilutions (1:5, 1:10, 1:20) of lentiviral vectors, as well as from cells that were not transduced (no LV). The custom Promega One4All kit extracted high-quality gDNA with absorbance ratios higher than 2, and both the DNA quality and yields meet requirements for analysis.