PCR Amplification
Introduction to PCR
The polymerase chain reaction (PCR) is a relatively simple technique that amplifies a DNA template to produce specific DNA fragments in vitro. Traditional methods of cloning a DNA sequence into a vector and replicating it in a living cell often require days or weeks of work, but amplification of DNA sequences by PCR requires only hours. While most biochemical analyses, including nucleic acid detection with radioisotopes, require the input of significant amounts of biological material, the PCR process requires very little. Thus, PCR can achieve more sensitive detection and higher levels of amplification of specific sequences in less time than previously used methods. These features make the technique extremely useful, not only in basic research, but also in commercial uses, including genetic identity testing, forensics, industrial quality control and in vitro diagnostics. Basic PCR is commonplace in many molecular biology labs where it is used to amplify DNA fragments and detect DNA or RNA sequences within a cell or environment. However, PCR has evolved far beyond simple amplification and detection, and many extensions of the original PCR method have been described. This chapter provides an overview of different types of PCR methods, applications and optimization.

Basic PCR
The PCR process was originally developed to amplify short segments of a longer DNA molecule (Saiki et al. 1985). A typical amplification reaction includes target DNA, a thermostable DNA polymerase, two oligonucleotide primers, deoxynucleotide triphosphates (dNTPs), reaction buffer and magnesium. Once assembled, the reaction is placed in a thermal cycler, an instrument that subjects the reaction to a series of different temperatures for set amounts of time. This series of temperature and time adjustments is referred to as one cycle of amplification. Each PCR cycle theoretically doubles the amount of targeted sequence (amplicon) in the reaction. Ten cycles theoretically multiply the amplicon by a factor of about one thousand; 20 cycles, by a factor of more than a million in a matter of hours.
Each cycle of PCR includes steps for template denaturation, primer annealing and primer extension. The initial step denatures the target DNA by heating it to 94°C or higher for 15 seconds to 2 minutes. In the denaturation process, the two intertwined strands of DNA separate from one another, producing the necessary single-stranded DNA template for replication by the thermostable DNA polymerase. In the next step of a cycle, the temperature is reduced to approximately 40–60°C. At this temperature, the oligonucleotide primers can form stable associations (anneal) with the denatured target DNA and serve as primers for the DNA polymerase. This step lasts approximately 15–60 seconds. Finally, the synthesis of new DNA begins as the reaction temperature is raised to the optimum for the DNA polymerase. For most thermostable DNA polymerases, this temperature is in the range of 70–74°C. The extension step lasts approximately 1–2 minutes. The next cycle begins with a return to 94°C for denaturation.
Each step of the cycle should be optimized for each template and primer pair combination. If the temperature during the annealing and extension steps are similar, these two steps can be combined into a single step in which both primer annealing and extension take place. After 20–40 cycles, the amplified product may be analyzed for size, quantity, sequence, etc., or used in further experimental procedures.
RT-PCR
Thermostable DNA polymerases used for basic PCR require a DNA template, and as such, the technique is limited to the analysis of DNA samples. Yet numerous instances exist in which amplification of RNA would be preferred. To apply PCR to the study of RNA, the RNA sample must first be converted to cDNA to provide the necessary DNA template for the thermostable polymerase (Figure 1). This process is called reverse transcription (RT), hence the name RT-PCR.
Reverse transcriptases (RTs) are RNA-directed DNA polymerases that were first identified as part of the retroviral life cycle (Temin and Mizutani, 1970, Baltimore, 1970). RTs catalyze the synthesis of a DNA copy (cDNA) of the target RNA molecules using a reverse transcription primer, dNTPs, and Mg2+ or Mn2+ as a cofactor. Reverse transcriptases have been adapted for use in a variety of in vitro applications including real-time and endpoint RT-PCR, labeled-cDNA probe generation and cDNA library construction. The ideal reverse transcriptase is robust (highly active under a variety of conditions) and converts all primed RNA within a sample to cDNA, regardless of its abundance, length or secondary structure.
The most characterized RTs used for molecular biology are the retroviral RTs: avian myeloblastosis virus (AMV) and Moloney murine leukemia virus (M-MLV or MuLV). Genetic engineering and development of proprietary RT-enhancing buffers have led to the commercial availability of new enzymes that offer superior performance over these naturally occurring RTs.
AMV and M-MLV reverse transcriptases are generally used to produce a DNA copy of the RNA template using either random primers, an oligo(dT) primer or sequence-specific primers. Some thermostable DNA polymerases (e.g., Tth DNA polymerase) possess a reverse transcriptase activity, which can be activated by using manganese instead of magnesium as a cofactor (Myers and Gelfand, 1991). After this initial reverse transcription step to produce the cDNA template, basic PCR is carried out to amplify the target sequence.
The quality and purity of the RNA template is crucial to the success of RT-PCR. Total RNA or poly(A)+ RNA can be used as the starting template, but both must be intact and free of contaminating genomic DNA. Specific capture of poly(A)+ RNA will enrich a targeted message so that less of the reverse transcription reaction is needed for subsequent amplification. The efficiency of the first-strand synthesis reaction, which can be related to the RNA quality, also will significantly affect amplification results.
GoScript™ Reverse Transcriptase is a formulation of M-MLV reverse transcriptase and optimized buffers designed for efficient and reproducible synthesis of first-strand cDNA from a full range of rare and abundant transcripts, even with difficult templates and in the presence of PCR inhibitors. GoScript is qualified for use in qPCR and is compatible with GoTaq® RT-qPCR Systems. GoScript is available in convenient mixes with either Oligo(dT) primers or random primers, as part of a complete kit, and as a stand-alone enzyme.
Figure 1. Diagram of RT-PCR process.
GoScript™ Reverse Transcriptase
Available as a standalone enzyme, a complete reverse transcription kit or as a master mix with Oligo(dT) or random primers for sensitive transcription of both high-copy and low-copy messages.
Hot-Start PCR
Hot-start PCR is a common technique to reduce nonspecific amplification due to assembly of amplification reactions at room temperature. At room temperature, PCR primers can anneal to template sequences that are not perfectly complementary. Since thermostable DNA polymerases have activity at these low temperatures (although in most cases the activity is less than 25%) the polymerase can extend misannealed primers. This newly synthesized region then acts as a template for primer extension and synthesis of undesired amplification products. However, if the reaction is heated to temperatures >60°C before polymerization begins, the stringency of primer annealing is increased, and synthesis of undesired PCR products is avoided or reduced.
Hot-start PCR also can reduce the amount of primer-dimer synthesized by increasing the stringency of primer annealing. At lower temperatures, PCR primers can anneal to each other via regions of complementarity, and the DNA polymerase can extend the annealed primers to produce primer dimer, which often appears as a diffuse band of approximately 50–100bp on an ethidium bromide-stained gel. The formation of nonspecific products and primer-dimer can compete for reagent availability with amplification of the desired product. Thus, hot-start PCR can improve the yield of specific PCR products.
To perform manual hot-start PCR, reactions are assembled on ice or at room temperature, but one critical component is omitted until the reaction is heated to 60–65°C, at which point the missing reagent is added. This omission prevents the polymerase from extending primers until the critical component is added at the higher temperature where primer annealing is more stringent. However, this method is tedious and increases the risk of contamination. A second, less labor-intensive approach involves the reversible inactivation or physical separation of one or more critical components in the reaction. For example, the magnesium or DNA polymerase can be sequestered in a wax bead, which melts as the reaction is heated to 94°C during the denaturation step, releasing the component only at higher temperatures (Carothers et al. 1989; Krishnan et al. 1991; Clark, 1988). The DNA polymerase also can be kept in an inactive state by binding to an oligonucleotide, also known as an aptamer (Lin and Jayasena, 1997; Dang and Jayasena, 1996) or an antibody (Scalice et al. 1994; Sharkey et al. 1994). This bond is disrupted at the higher temperatures, releasing the functional DNA polymerase. Finally, the DNA polymerase can be maintained in an inactive state through chemical modification (Moretti, T. et al 1998).
Hot-Start PCR Products
GoTaq® G2 Hot Start Taq is available as a standalone enzyme or master mix. In this formulation, the Taq polymerase is bound to a proprietary antibody that blocks activity. Activity is restored during initial denaturation, allowing hot-start PCR.
Long-Range PCR
Amplification of long DNA fragments is desirable for numerous applications such as physical mapping applications (Rose, 1991) and direct cloning from genomes. While basic PCR works well when smaller fragments are amplified, amplification efficiency (and therefore the yield of amplified fragments) decreases significantly as the amplicon size increases over 5kb. This decrease in yield can be attributed to the accumulation of truncated products, which are not suitable substrates for additional cycles of amplification. These products appear as smeared, as opposed to discrete, bands on a gel.
In 1994, Wayne Barnes (Barnes, 1994) and other researchers (Cheng et al. 1994) examined factors affecting polymerization across larger regions of DNA by thermostable DNA polymerases and identified key variables affecting the yield of longer PCR fragments. They devised an approach using a mixture of two thermostable polymerases to synthesize longer PCR products. The first polymerase lacks a 3′→5′ exonuclease (proofreading) activity; the second enzyme, present at a reduced concentration, contains a potent proofreading activity. Presumably, when the nonproofreading DNA polymerase (e.g., Taq DNA polymerase) misincorporates a dNTP, subsequent extension of the newly synthesized DNA either proceeds very slowly or stops completely. The proofreading polymerase (e.g., Pfu DNA polymerase or Tli DNA polymerase) serves to remove the misincorporated nucleotide, allowing the DNA polymerases to continue extension of the new strand.
Although the use of two thermostable DNA polymerases can significantly increase yield, other conditions can have a significant impact on the yield of longer PCR products (Cheng et al. 1995). Logically, longer extension times can increase the yield of longer PCR products because fewer partial products are synthesized. Extension times depend on the length of the target; times of 10–20 minutes are common. In addition, template quality is crucial. Depurination of the template, which is promoted at elevated temperatures and lower pH, will result in more partial products and decreased overall yield. In long PCR, denaturation time is reduced to 2–10 seconds to decrease depurination of the template. Additives, such as glycerol and dimethyl sulfoxide (DMSO), also help lower the strand-separation and primer-annealing temperatures, alleviating some of the depurination effects of high temperatures. Cheng et al. also found that reducing potassium concentrations by 10–40% increased the amplification efficiency of longer products (Cheng et al. 1995).
Not sure which Taq is best for your application? View our Taq DNA Polymerase Selection Guide.
Long PCR Master Mix
GoTaq® Long PCR Master Mix contains hot start Taq in a specially formulated mixture with a proprietary thermal stable proofreading polymerase. This optimized enzyme mixture allows efficient amplification of up to 40kb from lambda DNA or 30kb from human genomic DNA.
qPCR
Quantitative Endpoint PCR
PCR and RT-PCR are generally used in a qualitative format to evaluate biological samples. However, a wide variety of applications, such as determining viral load, measuring responses to therapeutic agents and characterizing gene expression, would be improved by quantitative determination of target abundance. Theoretically, this should be easy to achieve, given the exponential nature of PCR, because a linear relationship exists between the number of amplification cycles and the logarithm of the number of molecules. In practice, however, amplification efficiency is decreased because of contaminants (inhibitors), competitive reactions, substrate exhaustion, polymerase inactivation and target reannealing. As the number of cycles increases, the amplification efficiency decreases, eventually resulting in a plateau effect.
Normally, quantitative PCR requires that measurements be taken before the plateau phase so that the relationship between the number of cycles and molecules is relatively linear. This point must be determined empirically for different reactions because of the numerous factors that can affect amplification efficiency. Because the measurement is taken prior to the reaction plateau, quantitative PCR uses fewer amplification cycles than basic PCR. This can cause problems in detecting the final product because there is less product to detect.
To monitor amplification efficiency, many applications are designed to include an internal standard in the PCR. One such approach includes a second primer pair that is specific for a “housekeeping” gene (i.e., a gene that has constant expression levels among the samples compared) in the reaction (Gaudette and Crain, 1991; Murphy et al. 1990). Amplification of housekeeping genes verifies that the target nucleic acid and reaction components were of acceptable quality but does not account for differences in amplification efficiencies due to differences in product size or primer annealing efficiency between the internal standard and target being quantified.
The concept of competitive PCR—a variation of quantitative PCR—is a response to this limitation. In competitive PCR, a known amount of a control template is added to the reaction. This template is amplified using the same primer pair as the experimental target molecule but yields a distinguishable product (e.g., different size, restriction digest pattern, etc.). The amounts of control and test product are compared after amplification. While these approaches control for the quality of the target nucleic acid, buffer components and primer annealing efficiencies, they have their own limitations (Siebert and Larrick, 1993; McCulloch et al. 1995), including the fact that many depend on final analysis by electrophoresis.
Numerous fluorescent and solid-phase assays exist to measure the amount of amplification product generated in each reaction, but they often fail to discriminate amplified DNA of interest from nonspecific amplification products. Some of these analyses rely on blotting techniques, which introduce another variable due to nucleic acid transfer efficiencies, while other assays were developed to eliminate the need for gel electrophoresis yet provide the requisite specificity. Real-time PCR, which provides the ability to view the results of each amplification cycle, is a popular way of overcoming the need for analysis by electrophoresis.

qPCR Products
GoTaq® qPCR and RT-qPCR kits are available for dye-based or probe-based real-time PCR approaches. GoTaq qPCR Systems contain BRYT Green Dye, which provides maximum amplification efficiency and greater fluorescence than SYBR Green.
GoTaq® Probe Systems are ready-to-use master mixes that simplify reaction assembly for hydrolysis probe-based detection.
General Considerations for PCR Optimization
This discussion focuses on the use of Taq DNA polymerase in PCR, since this is the enzyme most commonly used in PCR. Many of these suggestions also apply when using other DNA polymerases. For more PCR tips, read this blog on the "Top Ten Tips for Successful PCR".
Magnesium Concentration
Magnesium is a required cofactor for thermostable DNA polymerases, and magnesium concentration is a crucial factor that can affect amplification success. Template DNA concentration, chelating agents present in the sample (e.g., EDTA or citrate), dNTP concentration and the presence of proteins all can affect the amount of free magnesium in the reaction. In the absence of adequate free magnesium, Taq DNA polymerase is inactive. Excess free magnesium reduces enzyme fidelity (Eckert and Kunkel, 1990) and may increase the level of nonspecific amplification (Williams, 1989; Ellsworth et al. 1993). For these reasons, researchers should empirically determine the optimal magnesium concentration for each target. To do so, set up a series of reactions containing 1.0–4.0mM Mg2+ in 0.5–1mM increments and visualize the results to determine which magnesium concentration produced the highest yield of product and the minimal amount of nonspecific product. The effect of magnesium concentration and the optimal concentration range can vary with the particular DNA polymerase. For example, the performance of Pfu DNA polymerase seems depend less on magnesium concentration, but when optimization is required, the optimal concentration is usually in the range of 2–6mM.
Many DNA polymerases are supplied with a magnesium-free reaction buffer and a tube of 25mM MgCl2 so that you can adjust the Mg2+ concentration to the optimal level for each reaction. Before assembling the reactions, be sure to thaw the magnesium solution completely prior to use and vortex the magnesium solution for several seconds before pipetting. Magnesium chloride solutions can form concentration gradients as a result of multiple freeze-thaw cycles, and vortex mixing is required to obtain a uniform solution. These two steps, though seemingly simple, eliminate the cause of many failed experiments.
Some scientists prefer to use reaction buffers that already contain MgCl2 at a final concentration of 1.5mM. It should be noted, however, that Hu et al. (1992) reported performance variability of reaction buffer solutions containing magnesium. The free magnesium changes of 0.6mM observed in their experiments dramatically affected amplification yields in an allele-specific manner. The authors found that heating the buffer at 90°C for 10 minutes restored the homogeneity of the solution. They postulated that magnesium chloride precipitates as a result of multiple freeze-thaw cycles.
Figure 2. Effects of magnesium concentration on amplification. Amplifications were performed using various Mg concentrations to demonstrate the effect on the amplification of a 1.8kb target luciferase gene. The reaction products were analyzed by agarose gel electrophoresis followed by ethidium bromide staining. Lane M, Promega pGEM® DNA Markers (Cat.# G1741); lane 1, 0mM Mg2+; lane 2, 0.5mM Mg2+; lane 3, 1mM Mg2+; lane 4, 1.5mM Mg2+; lane 5, 2mM Mg2+; lane 6, 2.5mM Mg2+; lane 7, 3mM Mg2+ and lane 8, 3.5mM Mg2+.
Buffer Considerations
Most reaction buffers consist of a buffering agent, most often a Tris-based buffer, and salt, commonly KCl. The buffer regulates the pH of the reaction, which affects DNA polymerase activity and fidelity. Modest concentrations of KCl will increase DNA polymerase activity by 50–60% over activities in the absence of KCl; 50mM KCl is considered optimal (Gelfand, 1989).
GoTaq® and GoTaq® G2 DNA Polymerase contain native Taq DNA polymerase in a proprietary formulation. It is supplied with 5X Green GoTaq® Reaction Buffer and 5X Colorless GoTaq® Reaction Buffer. The 5X Green GoTaq® Reaction Buffer contains blue and yellow dyes that separate during electrophoresis to monitor migration progress. The buffer also contains a compound that increases the density of the sample so that it will sink into the well of the agarose gel, allowing reactions to be directly loaded onto an agarose gel without the need for loading dye. The blue dye comigrates at the same rate as a 3–5kb DNA fragment in a 1% agarose gel. The yellow dye migrates at a rate faster than primers (<50bp) in a 1% agarose gel. The 5X Colorless GoTaq® Reaction Buffer and 5X Green GoTaq® Reaction Buffer have the same formulation, except for the dyes. The 5X Colorless GoTaq® Reaction Buffer is recommended for any applications where absorbance or fluorescence measurements of the PCR amplimer will be taken without prior cleanup. Both buffers are supplied at pH 8.5 and contain MgCl2 at a concentration of 7.5mM for a final concentration of 1.5mM.
GoTaq® Flexi and GoTaq® G2 Flexi DNA Polymerase are supplied with 5X Green GoTaq® Flexi Reaction Buffer and 5X Colorless GoTaq® Flexi Reaction Buffer. The compositions are identical to the 5X Green GoTaq® Reaction Buffer and 5X Colorless GoTaq® Reaction Buffer, except that the GoTaq® Flexi reaction buffers do not contain MgCl2. Instead, the GoTaq® Flexi DNA Polymerase is supplied with a tube of 25mM MgCl2 so that reactions can be supplemented with varying concentrations of magnesium.
Enzyme Concentration
We recommend using 1–1.25 units of Taq DNA polymerase in a 50μl amplification reaction. In most cases, this is an excess of enzyme, and adding more enzyme will not significantly increase product yield. In fact, increased amounts of enzyme increase the likelihood of generating artifacts associated with the intrinsic 5′→3′ exonuclease activity of Taq DNA polymerase, resulting in smeared bands in an agarose gel (Longley et al. 1990; Bell and DeMarini, 1991).
Pipetting errors are a frequent cause of excessive enzyme levels. Accurate dispensing of small volumes of enzyme solutions in 50% glycerol is difficult, so we strongly recommend preparing a reaction master mix, which requires a larger volume of each reagent, to reduce pipetting errors.
PCR Primer Design
PCR primers define the target region to be amplified and generally range in length from 15–30 bases. Ideally primers will have a GC-content of 40–60%. Avoid three G or C residues in a row near the 3′-end of the primer to minimize nonspecific primer annealing. Also, avoid primers with intra- or intermolecular complementary sequences to minimize the production of primer-dimer. Intramolecular regions of secondary structure can interfere with primer annealing to the template and should be avoided.
Ideally, the melting temperature (Tm), the temperature at which 50% of the primer molecules are annealed to the complementary sequence, of the two primers will be within 5°C so that the primers anneal efficiently at the same temperature. Primers can be designed to include sequences that are useful for downstream applications. For example, restriction enzyme sites can be placed at the 5′-ends of PCR primers to facilitate subsequent cloning of the PCR product, or a T7 RNA polymerase promoter can be added to allow in vitro transcription without the need to subclone the PCR product into a vector.
Template Quality
Successful amplification depends on DNA template quantity and quality. Reagents commonly used to purify nucleic acids (salts, guanidine, proteases, organic solvents and SDS) are potent inactivators of DNA polymerases. For example, 0.01% SDS will inhibit Taq DNA polymerase by 90%, while 0.1% SDS will inhibit Taq DNA polymerase by 99.9% (Konat et al. 1994). A few other examples of PCR inhibitors are phenol (Katcher and Schwartz, 1994), heparin (Beutler et al. 1990; Holodniy et al. 1991), xylene cyanol, bromophenol blue (Hoppe et al. 1992), plant polysaccharides (Demeke and Adams, 1992), and the polyamines spermine and spermidine (Ahokas and Erkkila, 1993). In some cases, the inhibitor is not introduced into the reaction with the nucleic acid template. A good example of this is an inhibitory substance that can be released from polystyrene or polypropylene upon exposure to ultraviolet light (Pao et al. 1993; Linquist et al. 1998).
If an amplification reaction fails and you suspect the DNA template is contaminated with an inhibitor, add the suspect DNA preparation to a control reaction with a DNA template and primer pair that has amplified well in the past . Failure to amplify the control DNA usually indicates the presence of an inhibitor. Additional steps to clean up the DNA preparation, such as phenol:chloroform extraction or ethanol precipitation, may be necessary.
Template Quantity
The amount of template required for successful amplification depends upon the complexity of the DNA sample. For example, of a 4kb plasmid containing a 1kb target sequence, 25% of the input DNA is the target of interest. Conversely, a 1kb target sequence in the human genome (3.3 × 109bp) represents approximately 0.00003% of the input DNA. Thus, approximately 1,000,000-fold more human genomic DNA is required to maintain the same number of target copies per reaction. Common mistakes include using too much plasmid DNA, too much PCR product or too little genomic DNA as the template. Reactions with too little DNA template will have low yields, while reactions with too much DNA template can be plagued by nonspecific amplification. If possible, start with >104 copies of the target sequence to obtain a signal in 25–30 cycles, but try to keep the final DNA concentration of the reaction ≤10ng/μl. When reamplifying a PCR product, the concentration of the specific PCR product is often not known. We recommend diluting the previous amplification reaction 1:10 to 1:10,000 before reamplification.
1μg of 1kb RNA = 1.77 × 1012 molecules
1μg of 1kb dsDNA = 9.12 × 1011 molecules
1μg of pGEM® Vector DNA = 2.85 × 1011 molecules
1μg of lambda DNA = 1.9 × 1010 molecules
1μg of E. coli genomic DNA = 2 × 108 molecules
1μg of human genomic DNA = 3.04 × 105 molecules
Cycling Parameters
The two most commonly altered cycling parameters are annealing temperature and extension time. The lengths and temperatures for the other steps of a PCR cycle do not usually vary significantly. However in some cases, the denaturation cycle can be shortened or a lower denaturation temperature used to reduce the number of depurination events, which can lead to mutations in the PCR products.
Primer sequence is a major factor that determines the optimal annealing temperature, which is often within 5°C of the melting temperature of the primers. Using an annealing temperature slightly higher than the primer Tm will increase annealing stringency and can minimize nonspecific primer annealing and decrease the amount of undesired products synthesized. Using an annealing temperature lower than the primer Tm can result in higher yields, as the primers anneal more efficiently at the lower temperature. We recommend testing several annealing temperatures, starting approximately 5°C below the Tm, to determine the best annealing conditions. In many cases, nonspecific amplification and primer-dimer formation can be reduced through optimization of annealing temperature, but if undesirable PCR products remain a problem, consider incorporating one of the many hot-start PCR methods.
Oligonucleotide synthesis facilities will often provide an estimate of a primer's Tm. The Tm also can be calculated using the Biomath Calculators. Numerous formulas exist to determine the theoretical Tm of nucleic acids (Baldino, Jr. et al. 1989; Rychlik et al. 1990). The formula below can be used to estimate the melting temperature for oligonucleotides:
Tm = 81.5 + 16.6 × (log10[Na+]) + 0.41 × (%G+C) – 675/n
where [Na+] is the molar salt concentration and n = number of bases in the oligonucleotide
Example. To calculate the melting temperature of a 22mer oligonucleotide with 60% G+C in 50mM KCl:
Tm = 81.5 + 16.6 × (log10[0.05]) + 0.41 × (60) – 675/22
= 81.5 + 16.6 × (–1.30) + 24.60 – 30.68 = 54°C
The length of the extension cycle, which may need to be optimized, depends on PCR product size and the DNA polymerase being used. In general, allow approximately 1 minute for every 1kb of amplicon (minimum extension time = 1 minute) for nonproofreading DNA polymerases and 2 minutes for every 1kb of amplicon for proofreading DNA polymerases. Avoid excessively long extension times, as they can increase the likelihood of generating artifacts associated with the intrinsic 5′→3′ exonuclease activity of Taq DNA polymerase (Longley et al. 1990; Bell and DeMarini, 1991).
PCR typically involves 25–35 cycles of amplification. The risk of undesirable PCR products appearing in the reaction increases as the cycle number increases, so we recommend performing only enough cycles to synthesize the desired amount of product. If nonspecific amplification products accumulate before sufficient amounts of PCR product can be synthesized, consider diluting the products of the first reaction and performing a second amplification with the same primers or primers that anneal to sequences within the desired PCR product (nested primers).
PCR Enhancers and Additives
Addition of PCR-enhancing agents can increase yield of the desired PCR product or decrease production of undesired products. There are many PCR enhancers, which can act through a number of different mechanisms. These reagents will not enhance all PCRs; the beneficial effects are often template- and primer-specific and will need to be determined empirically. Some of the more common enhancing agents are discussed below.
Addition of betaine, DMSO and formamide can be helpful when amplifying GC-rich templates and templates that form strong secondary structures, which can cause DNA polymerases to stall. GC-rich templates can be problematic due to inefficient separation of the two DNA strands or the tendency for the complementary, GC-rich primers to form intermolecular secondary structures, which will compete with primer annealing to the template. Betaine reduces the amount of energy required to separate DNA strands (Rees et al. 1993). DMSO and formamide are thought to aid amplification in a similar manner by interfering with hydrogen bond formation between two DNA strands (Geiduschek and Herskovits, 1961).
Some reactions that amplify poorly in the absence of enhancers will give a higher yield of PCR product when betaine (1M), DMSO (1–10%) or formamide (1–10%) are added. Concentrations of DMSO greater than 10% and formamide greater than 5% can inhibit Taq DNA polymerase and presumably other DNA polymerases as well (Varadaraj and Skinner, 1994).
In some cases, general stabilizing agents such as BSA (0.1mg/ml), gelatin (0.1–1.0%) and nonionic detergents (0–0.5%) can overcome amplification failure. These additives can increase DNA polymerase stability and reduce the loss of reagents through adsorption to tube walls. BSA also has been shown to overcome the inhibitory effects of melanin on RT-PCR (Giambernardi et al. 1998). Nonionic detergents, such as Tween®-20, NP-40 and Triton® X-100, have the added benefit of overcoming inhibitory effects of trace amounts of strong ionic detergents, such as 0.01% SDS (Gelfand and White, 1990). Ammonium ions can make an amplification reaction more tolerant of nonoptimal conditions. For this reason, some PCR reagents include 10–20mM (NH4)2SO4. Other PCR enhancers include glycerol (5–20%), polyethylene glycol (5–15%) and tetramethyl ammonium chloride (60mM).
Nucleic Acid Cross-Contamination
It is important to minimize cross-contamination between samples and prevent carryover of RNA and DNA from one experiment to the next. Use separate work areas and pipettors for pre- and post-amplification steps. Use positive displacement pipettes or aerosol-resistant tips to reduce cross-contamination during pipetting. Wear gloves, and change them often.
There are a number of techniques that can be used to prevent amplification of contaminating DNA. PCR reagents can be treated with isopsoralen, a photo-activated, cross-linking reagent that intercalates into double-stranded DNA molecules and forms covalent, interstrand crosslinks, to prevent DNA denaturation and replication. These inter-strand crosslinks effectively render contaminating DNA unamplifiable.
Treatment of PCR reagents with uracil-N-glycosylase (UNG), a DNA repair enzyme that hydrolyzes the base-ribose bond at uracil residues, eliminates one of the most common sources of DNA contamination: previously amplified PCR products. UNG treatment prevents replication of uracil-containing DNA by causing the DNA polymerase to stall at the resulting abasic sites. For UNG to be an effective safeguard against contamination, the products of previous amplifications must be synthesized in the presence of dUTP. This is easily accomplished by substituting dUTP for some or all of the dTTP in the reaction. Nonproofreading polymerases will readily incorporate dUTP into a PCR product, but proofreading polymerases incorporate dUTP much less efficiently (Slupphaug et al. 1993; Greagg et al. 1999; Lasken et al. 1996). Since dUTP incorporation has no noticeable effect on the intensity of ethidium bromide staining or electrophoretic mobility of the PCR product, reactions can be analyzed by standard agarose gel electrophoresis. While both methods are effective (Rys and Persing, 1993), UNG treatment has the advantage that both single-stranded and double-stranded DNA templates will be rendered unamplifiable (Longo et al. 1990).
Need bulk order options, unique formulations or custom sizes/formats of our products? Learn how our Custom Manufacturing team can partner with you to make it happen!
PCR Optimization Kit
Preformulated PCR master mix components to determine optimal PCR amplification conditions.
General Considerations for RT-PCR
Please also read General Considerations for PCR Optimization (above). Many of the important parameters discussed there also apply to RT-PCR. For a discussion of reverse transcriptases commonly used for RT-PCR, see the Thermostable Polymerases and Reverse Transcriptases section (below).
Template Considerations
For RT-PCR, successful reverse transcription depends on RNA integrity and purity. Procedures for creating and maintaining a ribonuclease-free (RNase-free) environment to minimize RNA degradation are described in Blumberg, 1987. The use of an RNase inhibitor (e.g., Recombinant RNasin® Ribonuclease Inhibitor) is strongly recommended. For optimal results, the RNA template, whether a total RNA preparation, an mRNA population or a synthesized RNA transcript, should be DNA-free to avoid amplification of contaminating DNA. The most commonly used DNA polymerases for PCR have no reverse transcriptase activity under standard reaction conditions, and thus, amplification products will be generated only if the template contains trace amounts of DNA with similar sequences.
Successful RT-PCR also depends on RNA quantity, which may need to be varied to determine the optimal amount. Excellent amplification results can be obtained with the Access and AccessQuick™ RT-PCR Systems using total RNA template levels in the range of 1pg–1μg per reaction (Figure 3) or poly(A)+ RNA template levels in the range of 1pg–100ng.
Figure 3. Amplification of a specific message in total RNA. RT-PCR amplifications containing the indicated amounts of mouse liver total RNA were performed using the Access RT-PCR System as described in the using oligonucleotide primers specific to the mouse β-actin transcript. The specific 540bp amplicon is indicated. Equivalent aliquots of each amplification reaction were separated on a 3% NuSieve®/ 1% agarose gel in 1X TAE buffer containing 0.5μg/ml ethidium bromide. Lanes M, 100bp DNA Ladder (Cat.# G2101).
Reverse Transcription Primer Design
Selection of an appropriate primer for reverse transcription depends on target mRNA size and the presence of secondary structure. For example, a primer that anneals specifically to the 3′-end of the transcript (a sequence-specific primer or oligo(dT) primer) may be problematic when reverse transcribing the 5′-ends of long mRNAs or molecules that have significant secondary structure, which can cause the reverse transcriptase to stall during cDNA synthesis. Random hexamers prime reverse transcription at multiple points along the transcript. For this reason, they are useful for either long mRNAs or transcripts with significant secondary structure.
Whenever possible, we recommend using a primer that anneals only to defined sequences in particular RNAs (sequence-specific primers) rather than to entire RNA populations in the sample (e.g., random hexamers or oligo(dT) primer). To differentiate between amplification of cDNA and amplification of contaminating genomic DNA, design primers to anneal to sequences in exons on opposite sides of an intron so that any amplification product derived from genomic DNA will be much larger than the product amplified from the target cDNA. This size difference not only makes it possible to differentiate the two products by gel electrophoresis but also favors the synthesis of the smaller cDNA-derived product (amplification of smaller fragments is often more efficient than that of long fragments).
Regardless of primer choice, the final primer concentration in the reaction is usually within the range of 0.1–1.0μM, but this may need to be optimized. We recommend using a final concentration of 1μM primer (50pmol in a 50μl reaction) as a starting point for optimization. More information on PCR primer design is provided in the PCR Primer Design section.
Cycle Parameters
Efficient first-strand cDNA synthesis can be accomplished in a 20- to 60-minute incubation at 37–45°C using AMV reverse transcriptase or at 37–42° for M-MLV reverse transcriptase. When using AMV RT we recommend using a sequence-specific primer and performing reverse transcription at 45°C for 45 minutes as a starting point. The higher reaction temperature will minimize the effects of RNA secondary structure and encourage full-length cDNA synthesis. First-strand cDNA synthesis with random hexamers and oligo(dT) primer should be conducted at room temperature (20–25°C) and 37°C, respectively.
The Access and AccessQuick™ RT-PCR Systems do not require RNA denaturation prior to initiation of the reverse transcription reaction. If desired, however, a denaturation step may be incorporated by incubating a separate tube containing the primers and RNA template at 94°C for 2 minutes. Do not incubate AMV reverse transcriptase at 94°C; it will be inactivated. The template/primer mixture then can be cooled to 45°C and added to the RT-PCR mix for the standard reverse transcription incubation at 45°C. Following the reverse transcription, we recommend a 2-minute incubation at 94°C to denature the RNA/cDNA hybrid, inactivate AMV reverse transcriptase and dissociate AMV RT from the cDNA. It has been reported that AMV reverse transcriptase must be inactivated to obtain high yields of amplification product (Sellner et al. 1992; Chumakov, 1994).
Most RNA samples can be detected using 30–40 cycles of amplification. If the target RNA is rare or if only a small amount of starting material is available, it may be necessary to increase the number of cycles to 45 or 50 or dilute the products of the first reaction and reamplify.
Thermostable Polymerases and Reverse Transcriptases
Thermostable DNA Polymerases
Prior to the use of thermostable DNA polymerases in PCR, researchers had to laboriously replenish the reaction with fresh enzyme (such as Klenow or T4 DNA polymerase) after each denaturation cycle. Thermostable DNA polymerases revolutionized and popularized PCR because of their ability to withstand the high denaturation temperatures. The use of thermostable DNA polymerases also allowed higher annealing temperatures, which improved the stringency of primer annealing.
Thermostable DNA polymerases can be used for either one-enzyme or two-enzyme RT-PCR (Myers and Gelfand, 1991; Chiocchia and Smith, 1997). For example, Tth DNA polymerase can act as a reverse transcriptase in the presence of Mn2+ for one-enzyme RT-PCR (Myers and Gelfand, 1991). All of the DNA polymerases mentioned below can be used to amplify first-strand cDNA produced by a reverse transcriptase, such as AMV RT, in two-enzyme RT-PCR.
Thermostable DNA polymerases can be divided into two groups: those with a 3′→5′ exonuclease (proofreading) activity, such as Pfu DNA polymerase, and those without the proofreading function, such as Taq DNA polymerase. These two groups have some important differences. Proofreading DNA polymerases are more accurate than nonproofreading polymerases due to the 3′→5′ exonuclease activity, which can remove a misincorporated nucleotide from a growing DNA chain. When the amplified product is to be cloned, expressed or used in mutation analysis, Pfu DNA polymerase is a better choice due to its high fidelity. However, for routine PCR, where simple detection of an amplification product is the goal, Taq DNA polymerase is the most commonly used enzyme because yields tend to be higher with a nonproofreading DNA polymerase.
Amplification with nonproofreading DNA polymerases results in the template-independent addition of a single nucleotide to the 3′-end of the PCR product, whereas the use of proofreading DNA polymerases results in blunt-ended PCR products (Clark, 1988; Hu, 1993). The single-nucleotide overhang can simplify the cloning of PCR products.
Proofreading DNA polymerases also are used in blends with nonproofreading DNA polymerases, or amino-terminally truncated versions of Taq DNA polymerase, to amplify longer stretches of DNA with greater accuracy than the nonproofreading DNA polymerase alone (Barnes, 1994; Cline et al. 1996).
Taq DNA Polymerase
Taq DNA polymerase is isolated from Thermus aquaticus and catalyzes the primer-dependent incorporation of nucleotides into duplex DNA in the 5′→3′ direction in the presence of Mg2+. The enzyme does not possess 3′→5′ exonuclease activity but has 5′→3′ exonuclease activity.
Taq DNA polymerase is suitable for most PCR applications that do not require a high-fidelity enzyme, such as detecting specific DNA or RNA sequences. The error rate of Taq DNA polymerase is approximately 1 × 10–5 errors/base, although the fidelity does depend somewhat on the reaction conditions. The fidelity is slightly higher at lower pH, lower magnesium concentration and relatively low dNTP concentration (Eckert and Kunkel, 1990; Eckert and Kunkel, 1991).
Taq DNA polymerase is commonly used to amplify PCR products of 5kb or less. PCR products in the range of 5–10kb can be amplified with Taq DNA polymerase but often require more optimization than smaller PCR products. For products larger than approximately 10kb, we recommend an enzyme or enzyme mix and reaction conditions that are designed for long PCR.
Taq DNA polymerase is a processive enzyme with an extension rate of >60 nucleotides/second at 70°C (Innis et al. 1988), so an extension step of 1 minute per 1kb to be amplified should be sufficient to generate full-length PCR products. The enzyme has a half-life of 40 minutes at 95°C (Lawyer et al. 1993). Because Taq DNA polymerase is a nonproofreading polymerase, PCR products generated with Taq DNA polymerase will contain a single-nucleotide 3′ overhang, usually a 3′ A overhang.
Pfu DNA Polymerase
Pfu DNA polymerase has one of the lowest error rates of all known thermophilic DNA polymerases used for amplification due to the high 3′→5′ exonuclease activity (Cline et al. 1996; Andre et al. 1997). For cloning and expressing DNA after PCR, Pfu DNA polymerase is often the enzyme of choice. Pfu DNA polymerase can be used alone to amplify DNA fragments up to 5kb by increasing the extension time to 2 minutes per kilobase. It is also used in blends with DNA polymerases lacking the proofreading function, such as Taq DNA polymerase, to achieve longer amplification products than with Pfu DNA polymerase alone (Barnes, 1994). However, the proofreading activity can shorten PCR primers, leading to decreased yield and increased nonspecific amplification.
Reverse Transcriptases
The discovery of reverse transcriptases, or RNA-dependent DNA polymerases, and their role in retrovirus infection (Baltimore, 1970; Temin and Mizutani, 1970) altered molecular biology’s central dogma of DNA→RNA→protein. Reverse transcriptases use an RNA template to synthesize DNA and require a primer for synthesis, like other DNA polymerases. For in vitro applications, the primer can be either oligo(dT), which hybridizes to the poly(A)+ tails of eukaryotic mRNAs, random hexamers, which prime synthesis throughout the length of the RNA template, or a sequence-specific primer, which hybridizes to a known sequence within the RNA template. Polymerization from a primer then proceeds as for DNA-dependent DNA polymerases. The commonly used reverse transcriptases, AMV reverse transcriptase, M-MLV reverse transcriptase and M-MLV reverse transcriptase, RNase H minus, perform the same reaction but at different optimum temperatures (AMV, 42°C; M-MLV, 37°C; and M-MLV RT, RNase H–, 42°C).
Some reverse transcriptases also possess intrinsic 3′- or 5′-exoribonuclease (RNase) activity, which is generally used to degrade the RNA template after first strand cDNA synthesis. Absence of the 5′-exoribonuclease (RNase H) activity may aid production of longer cDNAs (Berger et al. 1983).
Some DNA-dependent DNA polymerases also possess a reverse transcriptase activity, which can be favored under certain conditions. For example, the thermostable, DNA-dependent Tth DNA polymerase exhibits reverse transcriptase activity when Mn2+ is substituted for Mg2+ in a reaction.
AMV Reverse Transcriptase
AMV RT catalyzes DNA polymerization using template DNA, RNA or RNA:DNA hybrids (Houts et al. 1979). AMV reverse transcriptase is the preferred reverse transcriptase for templates with high secondary structure due to its higher reaction temperature (up to 58°C). AMV RT is used in a wide variety of applications including cDNA synthesis (Houts et al. 1979; Gubler and Hoffman, 1983), RT-PCR and rapid amplification of cDNA ends (RACE; Skinner et al. 1994). Although the high optimal temperature (42°C) makes it the enzyme of choice for cDNA synthesis using templates with complex secondary structure, its relatively high RNase H activity limits its usefulness for generation of long cDNAs (>5kb). For these templates, M-MLV RT or M-MLV RT, RNase H minus, may be a better choice.
M-MLV Reverse Transcriptase
M-MLV RT is a single-polypeptide, RNA-dependent DNA polymerase. The enzyme also has DNA-dependent DNA polymerase activity at high enzyme levels (Roth et al. 1985). M-MLV RT is used in a variety of applications, including cDNA synthesis, RT-PCR and RACE (Gerard, 1983). Its relatively low RNase H activity compared to AMV RT makes M-MLV RT the enzyme of choice for generating long cDNAs (>5kb) (Sambrook and Russell, 2001). However, for short templates with complex secondary structure, AMV RT or M-MLV RT, RNase H minus, may be a better choice due to their higher optimal temperatures. M-MLV RT is less processive than AMV RT, so more units of M-MLV RT may be required to generate the same amount of cDNA (Schaefer, 1995).
M-MLV Reverse Transcriptase, RNase H Minus
M-MLV reverse transcriptase, RNase H minus, is an RNA-dependent, 5′→3′ DNA polymerase that has been genetically altered to remove the associated ribonuclease H activity, which causes degradation of the RNA strand of an RNA:DNA hybrid (Tanese and Goff, 1988). The absence of RNase H activity makes M-MLV, RNase H minus, the enzyme of choice for generating long cDNAs (>5kb). However, for shorter templates with complex secondary structure, AMV reverse transcriptase may be a better choice because it can be used at higher reaction temperatures.
There are two forms of M-MLV, RNase H minus: the deletion mutant and the point mutant. As the names suggest, the deletion mutant had a specific sequence in the RNase H domain deleted, and the point mutant has a point mutation introduced in the RNase H domain. While the native M-MLV RT has a recommended reaction temperature of 37°C, the deletion and point mutants are more stable at higher temperatures and can be used at reaction temperatures of up to 50°C and 55°C, respectively, depending upon the reverse transcription primers used. The point mutant is often preferred over the deletion mutant because the point mutant has DNA polymerase activity comparable to that of the wildtype M-MLV enzyme, whereas the deletion mutant has a slightly reduced DNA polymerase activity compared to that of the wildtype enzyme (Figure 4).
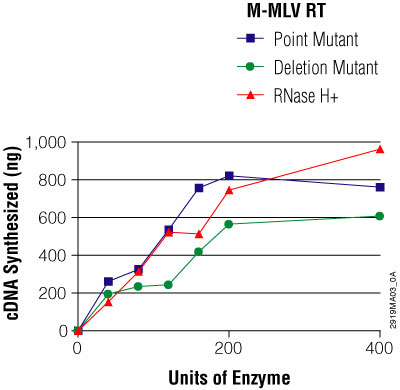
Figure 4. Comparison of the mass amount of total cDNA synthesized from 2μg of a 7.5kb RNA template by increasing amounts of three Promega M-MLV reverse transcriptases. Each first-strand cDNA reaction was performed using 2μg of a 7.5kb RNA template (1μl), 0.5μg of oligo(dT)15 primer (1μl) and 14μl water. The RNA and oligo(dT)15 primer were heated at 70°C for 5 minutes and cooled on ice for 5 minutes. Five microliters of M-MLV RT 5X Buffer, 1.25μl of 10μM dNTPs, 0.5μl of α-32P dCTP (10μCi/μl, 400Ci/mmol) and either 25, 50, 100, 150, 200 or 400 units of M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant; M-MLV Reverse Transcriptase, RNase H Minus, Deletion Mutant; or native M-MLV Reverse Transcriptase (RNase H+) was used in a final volume of 25μl. Reactions were incubated at 42°C for 60 minutes. TCA precipitations were performed, and first-strand cDNA yields were calculated.
GoScript® Reverse Transcriptase
Utilizes M-MLV reverse transcriptase enzyme and state-of-the-art buffer to drive robust, reliable cDNA synthesis of a full range of rare and abundant transcripts, even with difficult templates and in the presence of PCR inhibitors.
Example Protocols
Example Endpoint PCR Protocol- GoTaq® G2 DNA Polymerase
Materials Required
- GoTaq® G2 DNA Polymerase and Reaction Buffer (Cat.# M7841)
- PCR Nucleotide Mix (Cat.# C1141)
- Nuclease-Free Water (Cat.# P1193)
- upstream primer
- downstream primer
- template DNA
- mineral oil (optional)
- In a sterile, nuclease-free microcentrifuge tube, combine the following components on ice:
- If using a thermal cycler without a heated lid, overlay the reaction mix with 1–2 drops (approximately 50µl) of mineral oil to prevent evaporation during thermal cycling. Centrifuge the reactions in a microcentrifuge for 5 seconds.
- Place reactions into a thermal cycler that has been heated to 94–95°C and begin PCR.
| Component |
Volume |
Final Concentration |
|---|---|---|
|
5X Green or Colorless GoTaq® Reaction Buffer1 |
10µl
|
1X (1.5mM MgCl2)2
|
|
PCR Nucleotide Mix, 10mM |
1µl
|
0.2mM each dNTP
|
|
upstream primer |
Xµl
|
0.1–1.0µM
|
|
downstream primer |
Yµl
|
0.1–1.0µM
|
|
GoTaq® G2 DNA Polymerase (5u/µl) |
0.25µl
|
1.25u
|
|
template DNA |
Zµl
|
<0.5µg/50µl
|
|
Nuclease-Free Water |
to 50µl
|
|
1Thaw completely, and vortex thoroughly prior to use.
2More MgCl2 can be added to the reaction using 25mM MgCl2 Solution (Cat.# A3511)
Example RT Protocol: First-Strand cDNA Synthesis
The following procedure can be used to convert up to 5µg of total RNA or up to 500ng of poly(A) RNA into first-strand cDNA.
Materials Required
- GoScript® Reverse Transcription System (Cat.# M5000)
- High quality experimental RNA
- Mix and briefly centrifuge each component before use. Combine the following:
- Xµl Experimental RNA (up to 5µg/reaction)
- Primer [Oligo(dT)15 (0.5µg/reaction) and/or Random Primer (0.5µg/reaction) or gene-specific primer (10–20pmol/reaction)]
- Xµl Nuclease-Free Water to a final volume of 5µl
- Heat in a 70°C heat block for 5 minutes. Immediately chill in ice water for at least 5 minutes. Centrifuge 10 seconds in a microcentrifuge. Store on ice until reverse transcription mix is added.
- Prepare the reverse transcription reaction mix, 15µl for each cDNA reaction. Combine on ice, in the order listed.
- 4.0µl GoScript™ 5X Reaction Buffer
- 1.2–6.4µl MgCl2 (final concentration 1.5–5.0mM)1
- 1.0µl PCR Nucleotide Mix (final concentration 0.5mM each dNTP)2
- 20units Recombinant RNasin® Ribonuclease Inhibitor (optional)
- 1.0µl GoScript™ Reverse Transcriptase
- Xµl Nuclease-Free Water (to a final volume of 15µl)
- Combine 15µl of reverse transcription mix with 5µl of RNA and primer mix.
- Anneal in a heat block at 25°C for 5 minutes.
- Extend in a heat block at 42°C for up to one hour.
Reactions can be stopped at this point for analysis of the cDNA or may be frozen for long-term storage. - Inactivate Reverse Transcriptase: Before proceeding with qPCR, inactivate the reverse transcriptase in a heat block at 70°C for 15 minutes.
1Mg2+ concentration should be optimized to 1.5–5.0mM (MgCl2 provided at 25mM).
2If isotopic or nonisotopic incorporation is desired for monitoring first-strand cDNA synthesis, α[32P]-dCTP or other modified nucleotides may be supplemented into the PCR Nucleotide Mix.
Related PCR Products and Resources
Categories
Related Groups
Guides and Selectors
References
- Ahokas, H. and Erkkila, M.J. (1993) Interference of PCR amplification by the polyamines, spermine and spermidine. PCR Methods Appl. 3, 65–8.
- Andre, P. et al. (1997) Fidelity and mutational spectrum of Pfu DNA polymerase on a human mitochondrial DNA sequence. Genome Res. 7, 843–52.
- Arakawa, T. et al. (1996) Application of N-terminally truncated DNA polymerase from Thermus thermophilus (delta Tth polymerase) to DNA sequencing and polymerase chain reactions: Comparative study of delta Tth and wild-type Tth polymerases. DNA Res. 3, 87–92.
- Auer, T. et al. (1995) Properties of the 5′→3′ exonuclease/ribonuclease H activity of Thermus thermophilus DNA polymerase. Biochemistry 34, 4994–5002.
- Baldino, F., Jr. et al. (1989) High-resolution in situ hybridization histochemistry. Meth. Enzymol. 168, 761–77.
- Baltimore, D. (1970) RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 226, 1209–11.
- Barnes, W.M. (1994) PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc. Natl. Acad. Sci. USA 91, 2216–20.
- Bej, A.K. and Mahbubani, M.H. (1992) Applications of the polymerase chain reaction in environmental microbiology. PCR Methods Appl. 1, 151–9.
- Bell, D.A. and DeMarini, D.M. (1991) Excessive cycling converts PCR products to random-length higher molecular weight fragments. Nucl. Acids Res. 19, 5079.
- Berger, S.L. et al. (1983) Reverse transcriptase and its associated ribonuclease H: Interplay of two enzyme activities controls the yield of single-stranded complementary deoxyribonucleic acid. Biochemistry 22, 2365–72.
- Bernard, P.S. et al. (1998) Integrated amplification and detection of the C677T point mutation in the methylenetetrahydrofolate reductase gene by fluorescence resonance energy transfer and probe melting curves. Anal. Biochem. 255, 101–7.
- Beutler, E. et al. (1990) Interference of heparin with the polymerase chain reaction. Biotechniques 9, 166.
- Black, W.C. (1993) PCR with arbitrary primers: Approach with care. Insect Mol. Biol. 2, 1–6.
- Blumberg, D.D. (1987) Creating a ribonuclease-free environment. Meth. Enzymol. 152, 20–4.
- Brooks, E.M. et al. (1995) Secondary structure in the 3′ UTR of EGF and the choice of reverse transcriptases affect the detection of message diversity by RT-PCR. Biotechniques 19, 806–15.
- Carballeira, N. et al. (1990) Purification of a thermostable DNA polymerase from Thermus thermophilus HB8, useful in the polymerase chain reaction. Biotechniques 9, 276–81.
- Carothers, A.M. et al. (1989) Point mutation analysis in a mammalian gene: Rapid preparation of total RNA, PCR amplification of cDNA, and Taq sequencing by a novel method. Biotechniques 7, 494–9.
- Cheng, S. et al. (1994) Effective amplification of long targets from cloned inserts and human genomic DNA. Proc. Natl. Acad. Sci. USA 91, 5695–9.
- Cheng, S. et al. (1995) Template integrity is essential for PCR amplification of 20- to 30-kb sequences from genomic DNA. PCR Methods Appl. 4, 294–8.
- Chiocchia, G. and Smith, K.A. (1997) Highly sensitive method to detect mRNAs in individual cells by direct RT-PCR using Tth DNA polymerase. Biotechniques 22, 312–8.
- Chumakov, K.M. (1994) Reverse transcriptase can inhibit PCR and stimulate primer-dimer formation. PCR Methods Appl. 4, 62–4.
- Clark, J.M. (1988) Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucl. Acids Res. 16, 9677–86.
- Cline, J. et al. (1996) PCR fidelity of Pfu DNA polymerase and other thermostable DNA polymerases. Nucl. Acids Res. 24, 3546–51.
- Crockett, A.O. and Wittwer, C.T. (2001) Fluorescein-labeled oligonucleotides for real-time PCR: Using the inherent quenching of deoxyguanosine nucleotides. Anal. Biochem. 290, 89–97.
- Dang, C. and Jayasena, S.D. (1996) Oligonucleotide inhibitors of Taq DNA polymerase facilitate detection of low copy number targets by PCR. J. Mol. Biol. 264, 268–78.
- Demeke, T. and Adams, R. (1992) The effects of plant polysaccharides and buffer additives on PCR. Biotechniques 12, 332–4.
- Didenko, V. (2001) DNA probes using fluorescence resonance energy transfer (FRET): Designs and applications. Biotechniques 31, 1106–21.
- Eckert, K.A. and Kunkel, T.A. (1990) High fidelity DNA synthesis by the Thermus aquaticus DNA polymerase. Nucl. Acids Res. 18, 3739–44.
- Eckert, K.A. and Kunkel, T.A. (1991) DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1, 17–24.
- Edwards, J.B. et al. (1991) Oligodeoxyribonucleotide ligation to single-stranded cDNAs: A new tool for cloning 5′ ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucl. Acids Res. 19, 5227–32.
- Edwards, J.B. et al. (1993) Methods in Molecular Biology (Vol. 15), White, B.A., ed., Humana Press, Totowa, NJ.
- Ehrlich, H.A. et al. (1991) Recent advances in the polymerase chain reaction. Science 252, 1643–51.
- Ellsworth, D.L. et al. (1993) Artifactual variation in randomly amplified polymorphic DNA banding patterns. Biotechniques 14, 214–7.
- Frackman, S. et al. (2006) The Plexor™ Systems provide accurate quantitation in multiplex qPCR and qRT-PCR. Promega Notes 92, 10–13.
- Frohman, M.A. et al. (1988) Rapid production of full-length cDNAs from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85, 8998–9002.
- Fromont-Racine, M. et al. (1993) A highly sensitive method for mapping the 5′ termini of mRNAs. Nucl. Acids Res. 21, 1683–4.
- Gaensslen, R.E. et al. (1992) A polymerase chain reaction (PCR) method for sex and species determination with novel controls for deoxyribonucleic acid (DNA) template length. J. Forensic Sci. 37, 6–20.
- Gaudette, M.F. and Crain, W.R. (1991) A simple method for quantifying specific mRNAs in small numbers of early mouse embryos. Nucl. Acids Res. 19, 1879–84.
- Geiduschek, E.P. and Herskovits, T.T. (1961) Nonaqueous solutions of DNA. Reversible and irreversible denaturation in methanol. Arch. Biochem. Biophys. 95, 114–29.
- Gelfand, D.H. (1989) Taq DNA polymerase. In: PCR Technology: Principles and applications of DNA amplifications, Erlich, H.A., ed., Stockton Press, New York, 17–22.
- Gelfand, D.H. and White, T.J. (1990) In: PCR Protocols: A Guide to Methods and Applications, Innis, M.A., Gelfand, D.H., Sninsky, J.J. and White, T.J., eds, Academic Press, San Diego, CA, 129–41.
- Gerard, G.F. (1983) Enzymes of Nucleic Acid Synthesis and Modification: DNA Enzymes, CRC Press, Boca Raton, FL.
- Giambernardi, T.A. et al. (1998) Bovine serum albumin reverses inhibition of RT-PCR by melanin. Biotechniques 25, 564–6.
- Greagg, M.A. et al. (1999) A read-ahead function in archaeal DNA polymerases detects promutagenic template-strand uracil. Proc. Natl. Acad. Sci. USA 96, 9045–50.
- Gubler, U. and Hoffman, B.J. (1983) A simple and very efficient method for generating cDNA libraries. Gene 25, 263–9.
- Guo, B. and Bi, Y. (2002) Cloning PCR products: An overview. Methods Mol. Biol. 192, 111–9.
- Haase, A.T. et al. (1990) Amplification and detection of lentiviral DNA inside cells. Proc. Natl. Acad. Sci. USA 87, 4971–5.
- Holland, P.M. et al. (1991) Detection of specific polymerase chain reaction product by utilizing the 5′→3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 88, 7276–80.
- Holodniy, M. et al. (1991) Inhibition of human immunodeficiency virus gene amplification by heparin. J. Clin. Microbiol. 29, 676–9.
- Hoppe, B.L. et al. (1992) Gel-loading dyes compatible with PCR. Biotechniques 12, 679–80.
- Houts, G.E. et al. (1979) Reverse transcriptase from avian myeloblastosis virus. J. Virol. 29, 517–22.
- Hu, C.Y. et al. (1992) Effect of freezing of the PCR buffer on the amplification specificity: Allelic exclusion and preferential amplification of contaminating molecules. PCR Methods Appl. 2, 182–3.
- Hu, G. (1993) DNA polymerase-catalyzed addition of nontemplated extra nucleotides to the 3′ end of a DNA fragment. DNA Cell Biol. 12, 763–70.
- Hubank, M. and Schatz, D.G. (1994) Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucl. Acids Res. 22, 5640–8.
- Innis, M.A. et al. (1988) DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc. Natl. Acad. Sci. USA 85, 9436–40.
- Johnson, S.C. et al. (2004) A third base pair for the polymerase chain reaction: Inserting isoC and isoG. Nucl. Acids Res. 32, 1937–41.
- Kaledin, A.S. et al. (1981) Isolation and properties of DNA-polymerase from the extreme thermophilic bacterium Thermus flavus. Biokhimiia 46, 1576–84.
- Katcher, H.L. and Schwartz, I. (1994) A distinctive property of Tth DNA polymerase: Enzymatic amplification in the presence of phenol. Biotechniques 16, 84–92.
- Knoche, K. and Denhart, M. (2001) AccessQuick™ RT-PCR System: Simple, stable and sensitive. Promega Notes79, 12–4.
- Konat, G.W. et al. (1994) PCR Technology: Current Innovations, Griffin, H.G. and Griffin, A.M., eds, CRC Press, Inc. Boca Raton Florida.
- Krishnan, B.R. et al. (1991) Linear amplification DNA sequencing directly from single phage plaques and bacterial colonies. Nucl. Acids Res.19, 1153.
- Kurata, S. et al. (2001) Fluorescent quenching-based quantitative detection of specific DNA/RNA using a BODIPY® FL-labeled probe or primer. Nucl. Acids Res. 29, e34.
- Lasken, R.S. et al. (1996) Archaebacterial DNA polymerases tightly bind uracil-containing DNA. J. Biol. Chem. 271, 17692–6.
- Lawyer, F.C. et al. (1993) High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5′ to 3′ exonuclease activity. PCR Methods Appl. 2, 275–87.
- Lee, L.G. et al. (1993) Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucl. Acids Res. 21, 3761–6.
- Liang, P. and Pardee, A.B. (1992) Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257, 967–71.
- Lin, Y. and Jayasena, S.D. (1997) Inhibition of multiple thermostable DNA polymerases by a heterodimeric aptamer. J. Mol. Biol. 271, 100–11.
- Linquist, V. et al. (1998) UV irradiation of polystyrene pipets releases PCR inhibitors. Biotechniques 24, 50–2.
- Lisitsyn, N. et al. (1993) Cloning the differences between two complex genomes. Science 259, 946–51.
- Liu, X. and Gorovsky, M.A. (1993) Mapping the 5′ and 3′ ends of Tetrahymena thermophila mRNAs using RNA ligase mediated amplification of cDNA ends (RLM-RACE). Nucl. Acids Res. 21, 4954–60.
- Loh, E.Y. et al. (1989) Polymerase chain reaction with single-sided specificity: Analysis of T cell receptor delta chain. Science 243, 217–20.
- Longley, M.J. et al. (1990) Characterization of the 5′ to 3′ exonuclease associated with Thermus aquaticus DNA polymerase. Nucl. Acids Res. 18, 7317–22.
- Longo, M.C. et al. (1990) Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 93, 125–8.
- McClelland, M. and Welsh, J. (1994) DNA fingerprinting by arbitrarily primed PCR. PCR Methods Appl. 4, S59–65.
- McCulloch, R.K. et al. (1995) An evaluation of competitor type and size for use in the determination of mRNA by competitive PCR. PCR Methods Appl. 4, 219–26.
- Mezei, L.M. and Storts, D.R. (1994) Cloning PCR products. In: PCR Technology: Current Innovations, Griffin, H.G. and Griffin, A.M., eds., CRC Press, Boca Raton, FL, 21–7.
- Miller, K. and Storts, D. (1995) PCR ACCESS! A sensitive single-tube, two-enzyme system for RT-PCR. Promega Notes 53, 2–5.
- Miller, K. and Storts, D. (1996) An improved single buffer, two enzyme system for RT-PCR. J. NIH Res. 8(2), 48.
- Moretti, T. et al. (1998) Enhancement of PCR amplification yield and specificity using AmpliTaq Gold DNA polymerase. BioTechniques 25, 716–22.
- Moser, M.J. and Prudent, J.R. (2003) Enzymatic repair of an expanded genetic information system. Nucl. Acids Res. 31, 5048–53.
- Murphy, L.D. et al. (1990) Use of the polymerase chain reaction in the quantitation of mdr-1 gene expression. Biochemistry 29, 10351–6.
- Murray, V. (1989) Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucl. Acids Res. 17, 8889.
- Myers, T.W. and Gelfand, D.H. (1991) Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry 30, 7661–6.
- Nuovo, G.J. (1995) In situ PCR: Protocols and applications. PCR Methods Appl. 4, S151–67.
- Nuovo, G.J. et al. (1993) Importance of different variables for enhancing in situ detection of PCR-amplified DNA. PCR Methods Appl. 2, 305–12.
- Ohara, O. et al. (1989) One-sided polymerase chain reaction: The amplification of cDNA. Proc. Natl. Acad. Sci. USA 86, 5673–7.
- Pao, C.C. et al. (1993) Inhibition of in vitro enzymatic DNA amplification reaction by ultra-violet light irradiation. Mol. Cell Probes. 7, 217–9.
- Power, E.G.M. (1996) RAPD typing in microbiology—a technical review. J. Hosp. Infect. 34, 247–65.
- Rees, W.A. et al. (1993) Betaine can eliminate the base pair composition dependence of DNA melting. Biochemistry 32, 137–44.
- Rose, E.A. (1991) Applications of the polymerase chain reaction to genome analysis. FASEB J. 5, 46–54.
- Roth, M.J. et al. (1985) Purification and characterization of murine retroviral reverse transcriptase expressed in Escherichia coli. J. Biol. Chem. 260, 9326–35.
- Ruttimann, C. et al. (1985) DNA polymerases from the extremely thermophilic bacterium Thermus thermophilus HB-8. Eur. J. Biochem. 149, 41–6.
- Rychlik, W. et al. (1990) Optimization of the annealing temperature for DNA amplification in vitro. Nucl. Acids Res. 18, 6409–12.
- Rys, P.N. and Persing, D.H. (1993) Preventing false positives: Quantitative evaluation of three protocols for inactivation of polymerase chain reaction amplification products. J. Clin. Microbiol. 31, 2356–60.
- Saiki, R. et al. (1985) Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1350–4.
- Saiki , R.K. (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239, 487–91.
- Saluz, H. and Jost, J.P. (1989) A simple high-resolution procedure to study DNA methylation and in vivo DNA-protein interactions on a single-copy gene level in higher eukaryotes. Proc. Natl. Acad. Sci. USA 86, 2602–6.
- Sambrook, J. and Russell, D.W. (2001) Molecular Cloning: A Laboratory Manual, 3rd edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Scalice, E. et al. (1994) Monoclonal antibodies prepared against the DNA polymerase from Thermus aquaticus are potent inhibitors of enzyme activity. J. Immunol. Methods 172, 147–63.
- Schaefer, B.C. (1995) Revolutions in rapid amplification of cDNA ends: New strategies for polymerase chain reaction cloning of full-length cDNA ends. Anal. Biochem. 227, 255–73.
- Sellner, L.N. et al. (1992) Reverse transcriptase inhibits Taq polymerase activity. Nucl. Acids Res. 20, 1487–90.
- Sharkey, D. et al. (1994) Antibodies as thermolabile switches: High temperature triggering for the polymerase chain reaction. Biotechnology 12, 506–9.
- Sherrill, C.B. et al. (2004) Nucleic acid analysis using an expanded genetic alphabet to quench fluorescence. J. Am. Chem. Soc. 126, 4550–6.
- Siebert, P.D. and Larrick, J.W. (1993) PCR MIMICS: Competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques 14, 244–9.
- Skinner, T.L. et al. (1994) Three different calmodulin-encoding cDNAs isolated by a modified 5′-RACE using degenerate oligodeoxyribonucleotides. Gene 151, 247–51.
- Slupphaug, G. et al. (1993) Low incorporation of dUMP by some thermostable DNA polymerases may limit their use in PCR amplifications. Anal. Biochem. 211, 164–9.
- Staskus, K.A. et al. (1995) PCR in situ: New technologies with single-cell resolution for the detection and investigation of viral latency and persistence. In: The Polymerase Chain Reaction (PCR) for Human Viral Diagnosis. Clewley, J.P., ed., CRC Press, Boca Raton, FL, 21–40
- Tanese, N. and Goff, S.P. (1988) Domain structure of the Moloney murine leukemia virus reverse transcriptase: Mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc. Natl. Acad. Sci. USA 85, 1777–81.
- Temin, H. and Mizutani, S. (1970) RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 226, 1211–3.
- Thaker, V. (1999) In situ RT-PCR and hybridization techniques. Methods. Mol. Biol. 115, 379–402.
- Troutt, A.B. et al. (1992) Ligation-anchored PCR: A simple amplification technique with single-sided specificity. Proc. Natl. Acad. Sci. USA 89, 9823–5.
- Tyagi, S. et al. (1998) Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 16, 49–53.
- Varadaraj, K. and Skinner, D.M. (1994) Denaturants or cosolvents improve the specificity of PCR amplification of a G + C-rich DNA using genetically engineered DNA polymerases. Gene 140, 1–5.
- Williams, J.F. (1989) Optimization strategies for the polymerase chain reaction. Biotechniques 7, 762–9.
- Wittwer, C.T. et al. (2001) Real-time multiplex PCR assays. Methods 25, 430–42.
- Zhou, M.Y. et al. (1995) Universal cloning method by TA strategy. Biotechniques 19, 34–5.