Super Resolution Microscopy
Learn the basics of super resolution microscopy, including how it works, its applications and two common techniques: STED and STORM.
What is Super Resolution Microscopy?
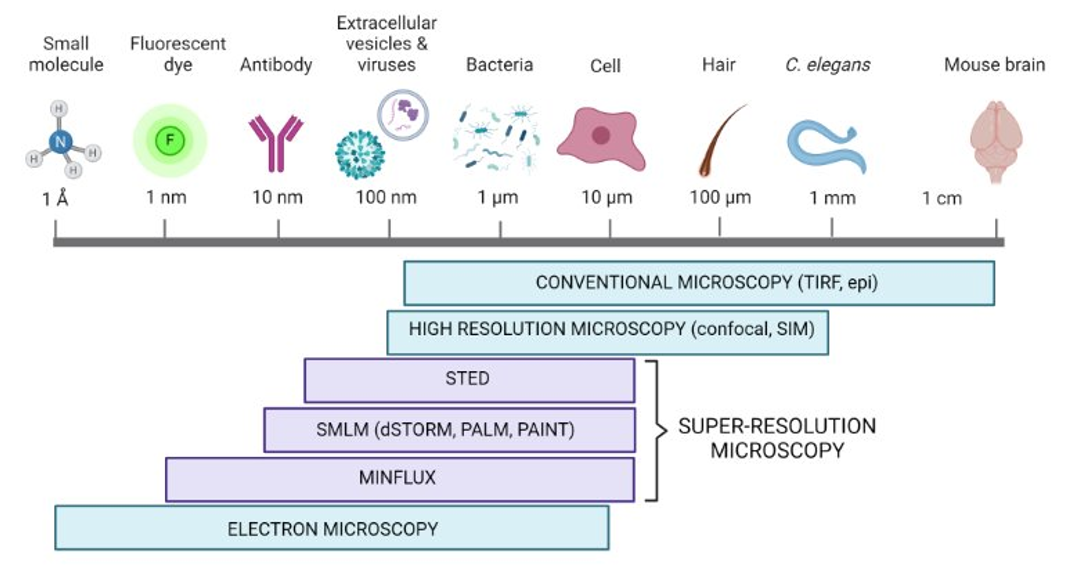
Traditional fluorescence microscopy can only image features down to a resolution of about 200 nanometers. Advances in modern imaging approaches, however, have broken the diffraction limit of light, giving rise to super resolution microscopy. Imaging methods using super resolution techniques allow researchers to observe structures at the nanoscale, providing exceptional resolution and insights into the molecular landscape. These techniques in combination with specific ligands (e.g, Janelia Fluor® HaloTag® Ligands) have revolutionized cell biology by allowing researchers to observe and analyze molecular processes with unprecedented detail.
Blinking: Light emitting species including organic dye molecules, fluorescent proteins, and semiconductor molecules switch between an “on” (fluorescent) and “off” (non-fluorescent) state. Blinking may be inherent to certain fluorophores or can be prompted by specific imaging conditions.
Triplet State: Refers to a specific energy state of a fluorophore. It is a higher energy state than the ground state but is characterized by a different spin state compared to the singlet state. The difference in spin state makes the return to ground state (and light emission) less likely or slower and can contribute to photobleaching.
Diffraction Limit: The limit of the resolving power of a conventional light microscope, which is determined by the wavelength of light and the numerical aperture of the microscope lens. In other words, this is the minimum size of a spot to which a lens can focus light.
Quantum Yield: The ratio of the number of protons emitted to the number of photons absorbed. Quantum yield describes the efficiency of a fluorophore to convert the excitation wavelength into fluorescence and is independent of instrument settings. In microscopy, quantum yield impacts a fluorophore’s brightness and photostability as well as the overall image quality.
Janelia Fluor® HaloTag® Ligands: Brighter Ligands, No-Wash Protocol
Photoactivatable Janelia Fluor® dyes offer improved brightness, quantum yield, and longer fluorescence lifetime, making them ideal for super-resolution microscopy applications. They are membrane permeable for use in single-molecule tracking experiments in live cells and are suitable for live imaging functional assays such as pulse-chase.
The HaloTag® platform allows the selection of various Janelia Fluor® dyes in different experiments, enabling complete flexibility to use the same construct in multiplex experiments on instruments with different filters.

What are the Different Types of Super Resolution Microscopy?
Stimulated emission depletion microscopy (STED) and stochastic optical reconstruction microscopy (STORM) are the two most common super resolution microscopy techniques. Learn more about these techniques in the comparison table and sections below.
| Modality | STED | STORM/dSTORM |
|---|---|---|
| Resolution | 30-70 nm | 10-55 nm |
| Illumination | Laser scanning confocal | Wide-field (epi/TIRF) |
| Acquisition Time | Short (seconds) | Long (minutes) |
| Post-acquisition Processing | No | Yes (centroid identification) |
| Data Size | Small (1 image) | Large (many frames) |
| Live Cell Compatible | Yes | No |
| Other Concerns | Photobleaching | Over/under labeling artifacts |
How Super Resolution Microscopy Is Used Across Research Applications
Cell Biology
Super resolution microscopy techniques, such as STED and STORM, can reveal structures within cells at a much finer detail than traditional fluorescence microscopy. This includes the organization of organelles, cytoskeletal structures, and membrane complexes. This improved spatial distribution of molecules within complexes provides insights into the stoichiometry and organization of molecular assemblies.
Neuroscience
STORM and STED can be used to map the distribution and organization of synaptic proteins, dendritic spines, and neural networks at an unprecedented resolution, aiding in the understanding of synaptic function and neural circuitry.
Pathogen-Host Interactions
STORM allows for the detailed study of the interactions between pathogens (such as viruses and bacteria) and their host cells, providing insights into the mechanisms of infection and immune response at the molecular level.
Direct Imaging (STED Specific)
Unlike techniques that rely on post-acquisition computational reconstruction (such as STORM), STED provides real-time super-resolution images. This direct imaging capability simplifies the process and reduces potential artifacts introduced during image reconstruction.
What is STED Microscopy?
Stimulated Emission Depletion (STED) microscopy is an advanced fluorescence microscopy technique that surpasses the diffraction limit of conventional optical microscopy, STED microscopy exploits the principles of stimulated emission to achieve nanometer-scale resolution.
How Does STED Microscopy Work?
The fundamental principle of STED microscopy involves two laser beams: an excitation laser and a depletion laser. The excitation laser initially excites fluorophores in the specimen to a higher energy state. The depletion laser, typically donut-shaped, is then employed to stimulate the excited fluorophores back to their ground state, except for those in a very small central region where the intensity of the depletion laser is zero. This selective depletion effectively reduces the fluorescence-emitting area, thus confining the emission to a sub-diffraction volume. By scanning this focal spot across the sample, high-resolution images are constructed.
High Resolution
STED can achieve a lateral resolution of 20-50 nanometers, significantly surpassing the diffraction limit of conventional light microscopy. This allows for the detailed visualization of subcellular structures.
Real-Time Imaging
STED provides real-time super-resolution imaging without the need for post-acquisition image reconstruction. This makes STED more suitable for live-cell imaging and studying dynamic processes within cells.
Direct Imaging
Unlike techniques that rely on post-acquisition computational reconstruction (such as STORM), STED provides real-time super-resolution images. This direct imaging capability simplifies the process and reduces potential artifacts introduced during image reconstruction.
Quantitative Analysis
STED provides quantitative information about the spatial distribution and organization of molecules within a sample, aiding in the understanding of molecular complexes and cellular structures.
Complexity and Cost
STED microscopy requires both advanced technology and maintenance, meaning it has a high cost for entry into this application. Likewise, the initial cost of acquiring a STED microscope is often significantly higher than conventional microscopy systems due to the advanced hardware and software required.
High Phototoxicity and Photobleaching
High-intensity light is required for the depletion process, which can lead to detrimental effects on both fixed and live samples. Increased light intensity accelerates photobleaching, the process in which fluorescent molecules lose their ability to emit light throughout the duration of an imaging session.
Interested in pushing the boundaries of what's possible with microscopy?
Hear from our experts on how the newest advancements in HaloTag® Technology enable microscopy imaging techniques.
Webinar: Advancing Cellular Imaging and Enhanced Tagging Technologies
Webinar: Using HaloTag® Technology and Janelia Fluor® Dyes for Live Cell Single-Molecule Imaging
What is STORM Microscopy?
Stochastic Optical Reconstruction Microscopy (STORM) is a super-resolution microscopy technique that surpasses the diffraction limit of visible light, enabling the visualization of biological structures at the nanometer scale. STORM utilizes the principles of single-molecule localization to achieve high spatial resolution.
How Does STORM Microscopy Work?
The core principle of STORM microscopy relies on the precise localization of individual fluorophores. By temporally separating the activation and imaging of single fluorophores within a densely labeled sample, STORM constructs high-resolution images through iterative cycles of activation, imaging, and localization. The main components and mechanisms of STORM are described below:
- Fluorophores: Specially designed photoswitchable or photoactivatable fluorophores are crucial for STORM. These fluorophores can be toggled between a fluorescent and a non-fluorescent state using specific wavelengths of light.
- Activation and Imaging: The fluorophores are stochastically activated in sparse subsets, ensuring that only a few emit fluorescence at any given time. This prevents overlap of the emission spots, allowing for precise localization.
- Single-Molecule Localization: Each activated fluorophore’s position is determined with high precision by fitting the point spread function (PSF) of its emitted light to a Gaussian profile. The coordinates of individual fluorophores are then recorded.
- Image Reconstruction: After many cycles of activation and imaging, a super-resolution image is reconstructed from the accumulated positions of thousands to millions of single fluorophores.
High Spatial Resolution
Achieves lateral resolution of 10–30 nm and axial resolution of 50–60 nm, which is among the highest of all super resolution methods.
Single-Molecule Detection
STORM relies on the precise localization of individual fluorescent molecules and enables the detection and analysis of individual proteins all the way down to single molecule resolution.
Quantitative Data
The technique provides quantitative information about the number and spatial distribution of molecules within a sample, aiding in understanding molecular stoichiometry and the organization of molecular complexes.
Complex Sample Preparation
Due to the requirement of photoswitchable fluorophores, a limited number of dyes and fluorescent proteins are compatible with this technique.
Slow Image Acquisition
STORM is highly time-intensive, as it relies on capturing many sequential images to reconstruct a final super-resolved image. This also makes this technique less suitable for dynamic processes or live-cell imaging.
Additional Resources
Imaging
Learn about various imaging techniques, including live cell imaging, in vivo imaging, bioluminescence imaging and fluorescence microscopy.
Fluorescence Imaging
Learn the basics of fluorescence microscopy, including its applications and advantages.
Bioluminescence Imaging
Learn about bioluminescence imaging in whole animals and in live cells.