GPCR Research Solutions
G protein-coupled receptors (GPCRs) represent one of the most important classes of drug targets. Discover how to measure the response along each step of the GPCR signaling cascade with easy-to-use, bioluminescence-based assays.
We would be happy to help solve challenges in your research—just ask us!

Learn how NanoBRET® technology is used to study the effects of drugs on GPCRs.
GPCRs: Key Drivers in Drug Discovery
G-protein-coupled receptors (GPCRs) are a diverse family of cell surface proteins critical for cellular communication. They regulate vital physiological processes and are the target of nearly 35% of all approved drugs. Their central role in disease pathways makes GPCRs essential for drug discovery, offering opportunities to develop treatments for cancer, cardiovascular, and neurological disorders.

Ligand Binding
Ligand binding to GPCRs is a critical step in drug discovery, providing insights into receptor activation and signaling. Measuring ligand affinity and residence time is essential for understanding how ligands interact with receptors, which influence drug efficacy and duration of action. These parameters guide the development of more effective and selective therapeutics.
Measure Ligand Binding in Live Cells
NanoBRET® target engagement is a highly sensitive and quantitative method for measuring ligand binding. This involves labeling the GPCR with a HiBiT tag (via transient transfection or CRISPR/Cas9) and labeling the ligand with a fluorophore. When the ligand binds to the GPCR, their close proximity generates energy transfer, resulting in a fluorescence signal that enables precise quantification.

Schematic of assays utilizing the HiBiT/LgBiT reporter for monitoring ligand engagement via BRET. See details in this publication: The luminescent HiBiT peptide enables selective quantitation of G protein–coupled receptor ligand engagement and internalization in living cells.

Saturation binding of a Clozapine NanoBRET® tracer to five GPCRs in the α-adrenergic receptor family. See details in this publication: An integrated approach toward NanoBRET tracers for analysis of GPCR ligand engagement.
GPCR Internalization
GPCR internalization is a key regulatory process that controls receptor availability and signaling dynamics. After ligand binding and activation, receptors are internalized into endosomes, where they can either be degraded or recycled back to the cell surface. Recycling restores GPCR availability, ensuring sustained cellular responsiveness and signaling balance, making it a critical aspect of drug discovery and therapeutic design.
Measure GPCR Internalization in Real Time
The Nano-Glo® HiBiT Extracellular Detection System is an easy way to quantify proteins expressed on the cell surface in real time. This antibody-free HiBiT tag-based approach allows you to measure receptor internalization and subsequent recycling and eliminate the variability associated with antibody-based methods. See details in this white paper: Quantifying Percent Surface Expression Using Bioluminescent Detection of the HiBiT Protein Tag

Schematic of assays utilizing the HiBiT/LgBiT reporter for monitoring ligand-induced GPCR internalization. See details in this publication: The luminescent HiBiT peptide enables selective quantitation of G protein–coupled receptor ligand engagement and internalization in living cells.

Isoproterenol-dependent internalization monitored with the Nano-Glo® HiBiT Extracellular Detection System. The HiBiT tag was introduced into the β2-AR gene in HEK293 cells using CRISPR gene editing.
β-Arrestin
β-Arrestins regulate GPCR signaling by desensitizing receptors, mediating internalization, and initiating G-protein-independent pathways. Their interaction with GPCRs is key to controlling signaling dynamics and receptor trafficking. Monitoring GPCR-β-arrestin interaction provides critical insights into receptor function and is essential for developing biased ligands that target specific signaling pathways in drug discovery.
Monitor GPCR-β-Arrestin Interaction in Real-Time
NanoBiT® Technology enables real-time, quantitative analysis of GPCR-β-arrestin protein-protein interactions by generating a luminescent signal upon complex formation. See an example in this blog: Bioassay for Cannabinoid Receptor Agonists Designed with NanoBiT® Techology



Visualize GPCR-β-Arrestin Interaction With Bioluminescence Imaging
The GloMax® Galaxy Bioluminescence Imager allows you to visualize GPCR-β-arrestin PPIs at the cellular level. It differentiates responsive and non-responsive cells while revealing interaction rates within cell populations, transforming data collection into a visual exploration of real-time biological processes. See an example in this blog: Visualize Protein:Protein Interactions with Bioluminescence Imaging

Treatment with Fractalkine leads to an increase in NanoBiT® luminescent signal, indicative of CX3CR1:AARB2 interaction. Localization shifts from predominantly membrane to intracellular puncta, suggesting receptor internalization. See details.
cAMP Assays
Secondary messengers, such as cAMP and IP3, are key molecules generated downstream of GPCR activation. Upon ligand binding, GPCRs activate G-proteins, which stimulate enzymes like adenylyl cyclase or phospholipase C to produce these messengers. Secondary messengers amplify the signal, triggering diverse cellular responses, such as changes in gene expression, metabolism, or calcium signaling, making them central to understanding GPCR-mediated pathways and drug effects.
High-Throughput Screening of cAMP
Lumit® Technology offers a rapid, no-wash bioluminescent method for measuring cAMP. This homogeneous assay simplifies workflows, minimizes interference, and delivers accurate, high-throughput results in minutes.


Measure Intracellular cAMP in Cell Lysates
The cAMP-Glo™ Max Assay is a sensitive, reproducible bioluminescent assay for measuring intracellular cAMP. Compatible with diverse samples and high-throughput formats, it offers extended signal stability and minimizes interference for reliable results.
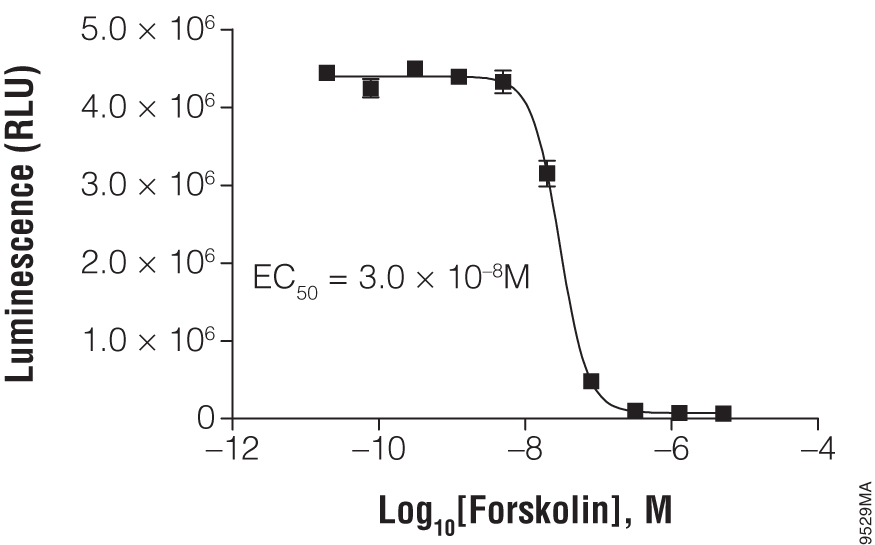
Titration of forskolin using cAMP-Glo™ Max Assay. In a white, clear-bottom, 384-well plate, 2,000 HEK293 cells were exposed to the indicated concentration of forskolin.

Using cAMP-Glo™ Max Assay to determine the IC50 value of SCH23390 in D1 receptor-expressing HEK293 cells. Cells were treated with the antagonist, SCH23390, in the presence of 100nM agonist, SKF38393.
Real-Time Detection of cAMP in Live Cells
GloSensor™ technology provides a platform of flexible luciferase-based biosensors for real-time detection of signaling events in live cells with sensitivity, linearity and specificity. Its broad dynamic range allows detection of up to 500-fold changes in light output. Extreme sensitivity allows detection of Gi-coupled receptor activation or inverse agonist activity without artificial stimulation by compounds (e.g., forskolin).
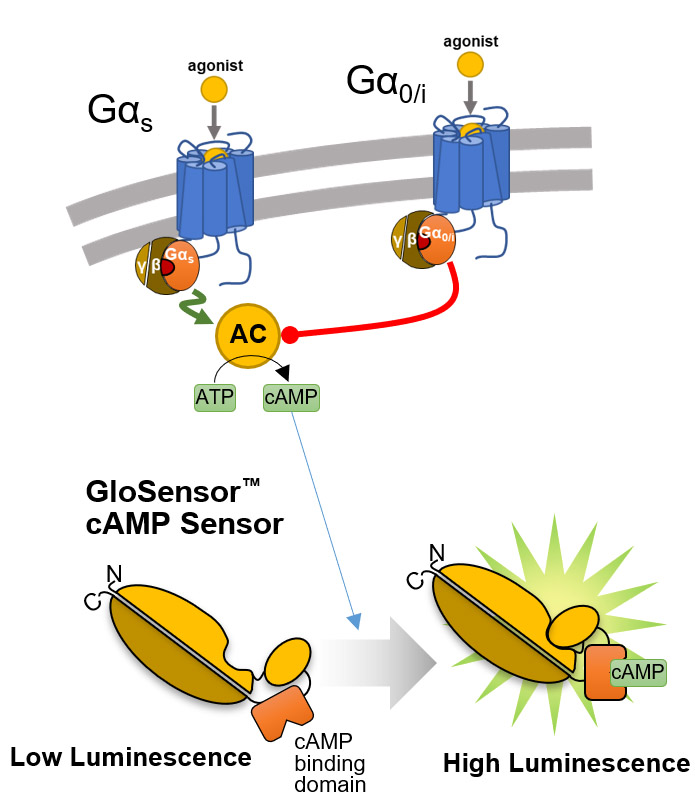
Overview of the GloSensor™ cAMP Assay. Genetically encoded biosensor variants contain cAMP binding domains fused to mutant forms of Photinus pyralis luciferase. Upon binding to cAMP, conformational changes occur that promote large increases in light output.
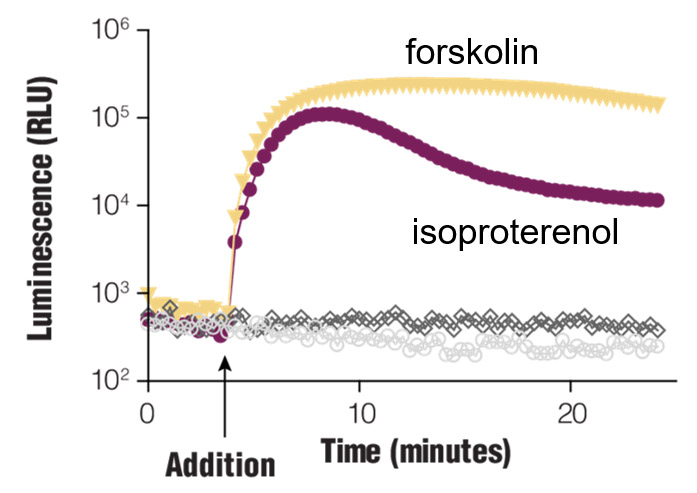
Real-time Gαs monitoring. HEK293 cells were transiently transfected with pGloSensor™-22F cAMP Plasmid and treated with compounds as shown.
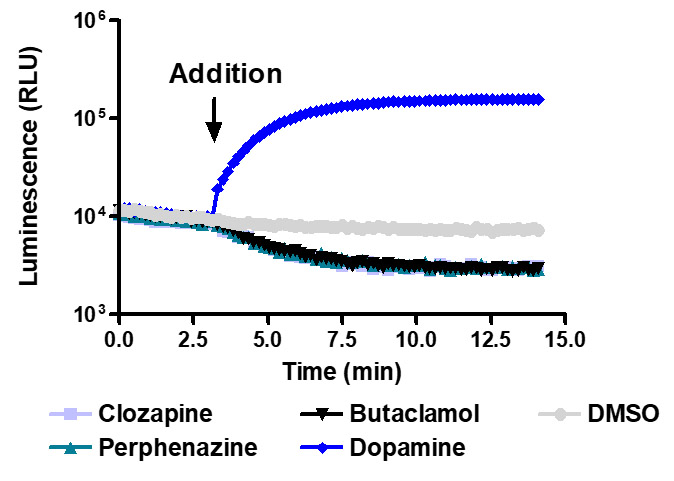
Sensitive enough to detect inverse agonists without forskolin. HEK293 cells expressing Dopamine D1 receptor were transiently transfected with pGloSensor™-22F cAMP Plasmid and treated with 10µM compounds at 28°C.
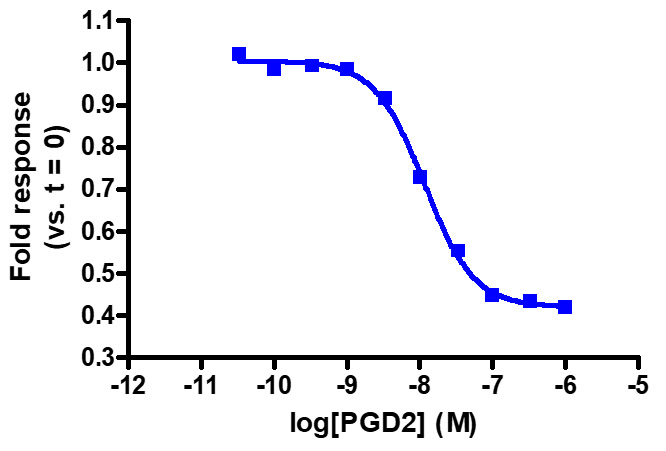
Sensitive enough to detect Gαi activity without forskolin. HEK293 cells expressing GPR44 were transiently transfected with pGloSensor™-22F cAMP Plasmid and treated with prostaglandin D2 (PGD2).
Gene Expression
GPCR signaling regulates gene expression by activating intracellular pathways that influence transcription. Upon ligand binding, GPCRs trigger cascades involving secondary messengers and kinases, which modulate the activity of transcription factors. This process enables cells to adapt to their environment and regulate essential functions such as growth, differentiation, and metabolism.
Measure GPCR Function With Bioassays
Our bioassays use engineered cells that couple receptor activation at the cell surface to a specific response element linked to a luciferase reporter. When a ligand binds to the receptor, intracellular signaling pathways are activated, driving the expression of luciferase. The resulting luminescent signal provides a sensitive, quantitative readout of receptor activity, enabling precise measurement of GPCR function and downstream signaling.
Contact our Tailored R&D Solutions Team about assays for GLP-1, GIP, and glucagon receptors. We can even help you create a bioassay for your GPCR of interest!


Ranked potency of GLP-1R agonists using the GLP-1 Bioassay. The following EC50 values were determined: Semaglutide: 52pM, Duraglutide: 38pM, Liraglutide: 20pM, Exendin: 6pM, Lixisenatide: 5pM.
Measure GPCR-Activated Transcription Factors With Reporter Vectors

Services to Support GPCR Research
Need customized tools or expert guidance to support your GPCR research? Our Tailored R&D Solutions Team is here to help! Our services include:
- Assay Development and Optimization: Assays designed for your research needs (e.g., GPCR ligand binding, receptor internalization, and secondary messenger production).
- Custom Bioassay Creation: Engineering cell lines to link GPCR activation to luciferase-based reporters for precise measurement of receptor activity and downstream gene expression.
- Advanced Internalization Studies: Tools for studying GPCR internalization and receptor recycling to understand signaling regulation.
- Secondary Messenger and Kinase Pathway Analysis: Support for assays measuring GPCR-mediated production of cAMP, IP3, and downstream activation of kinases.
- Comprehensive Support: Collaboration with our scientists to adapt technologies for novel applications in GPCR biology or other receptor-based studies.
Explore our easy-to-use, bioluminescence-based assays for GPCR research.