Antibody-Drug Conjugate (ADC) Discovery and Development
Enhance your antibody-drug conjugate (ADC) research with our suite of technologies tailored to support every phase of ADC development. From the early stages of concept exploration to detailed in vitro efficacy evaluations, our tools are designed to offer essential insights that empower your research. Streamline your workflow with advanced on-bead purification and conjugation processes, delve into the specifics of internalization efficiency, and accurately assess your ADC's functionality and specificity.
Let us know how we can help accelerate your path to discovery!

See how innovative assays like CellTiter-Glo® and RealTime-Glo™ streamline workflows, enhance insights, and drive advancements in ADC research.
What are ADCs?
Antibody-drug conjugates (ADCs) represent a significant advancement in targeted drug delivery technology, particularly in the field of oncology. The concept of ADCs hinges on three critical components: the antibody, the linker, and the payload. The antibody is engineered to recognize and bind to a specific antigen, typically overexpressed on the surface of cancer cells. The linker, which can be cleavable or non-cleavable, securely attaches the cytotoxic drug (payload) to the antibody. This linker is designed to be stable in the bloodstream but to release the drug once the ADC has been internalized by the cancer cell, thus delivering the lethal payload directly to the tumor. Discovery and development of ADCs involves crucial steps from antibody selection and ADC internalization to payload function assessment.
ADCs combine the specific targeting capabilities of monoclonal antibodies with the potent cytotoxic effects of small molecule drugs, creating a powerful therapeutic tool designed to selectively target and kill tumor cells while minimizing damage to healthy tissue. We offer analytical tools that examine both the Fc effector function, which mediates immune responses, and the payload function, responsible for direct tumor cell killing. These tools, including Fc receptor characterization and cytotoxicity assays, can help evaluate the overall efficacy and safety of ADC therapies.
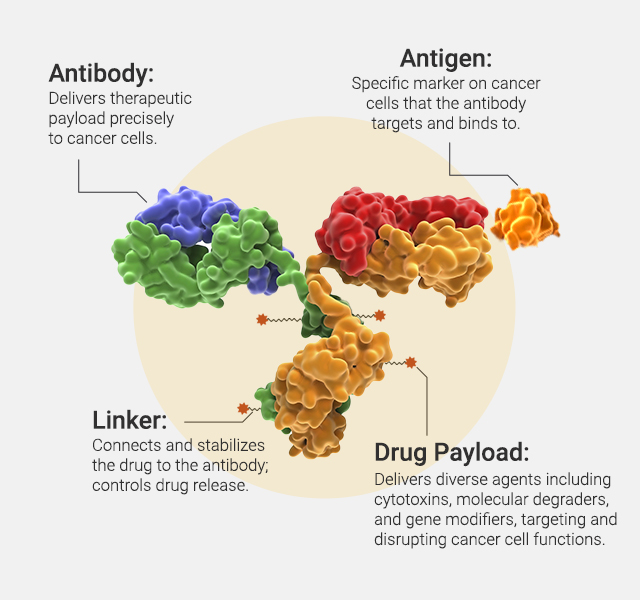
Engineering
On-bead Conjugation and Purification
Magne® Protein G and Magne® Protein A Beads are designed for the efficient and selective purification of antibodies. For ADC development, this technology facilitates the isolation of high-purity antibodies from complex biological mixtures and allows on-bead conjugation. The use of magnetic beads allows for a straightforward, scalable, and rapid conjugation and purification process. Explore our high-throughput Maxwell® Antibody Capture System, compatible with KingFisher® magnetic particle processors or liquid handlers in your lab. Our Field Support team, with expertise on HT platforms of all kinds, can program this chemistry for success in your lab.
See data in this publication: On-bead antibody-small molecule conjugation using high-capacity magnetic beads.
Mass Spectrometry
Mass spectrometry (MS) is a critical tool for ADC research for its precision in characterizing, quantifying, and ensuring that Critical Quality Attributes (CQA) of these complex therapeutics are met. MS can be used to:
- confirm sequence of biotherapeutic protein, including ADC conjugation site
- reveal drug-to-antibody ratios
- accurately measure ADC concentrations in biological samples (preclinical and clinical)
- assess ADC stability and degradation
- ensure each ADC batch meets stringent quality standards and lot-to-lot consistency
- monitor host cell protein impurities
Learn more about our solutions for MS, including proteases and reference reagents.
See data in this poster: Characterization of Therapeutic Antibodies with a ProAlanase
Proteases for ADC Applications
Internalization
pHAb Reactive Dyes enable ADC researchers to precisely track antibody internalization into cells. These dyes fluoresce in acidic conditions, like those in endosomes and lysosomes, offering a quantitative measure of ADC delivery efficiency. This aids in evaluating and enhancing ADC therapeutic potential.
See more data in this publication: Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye
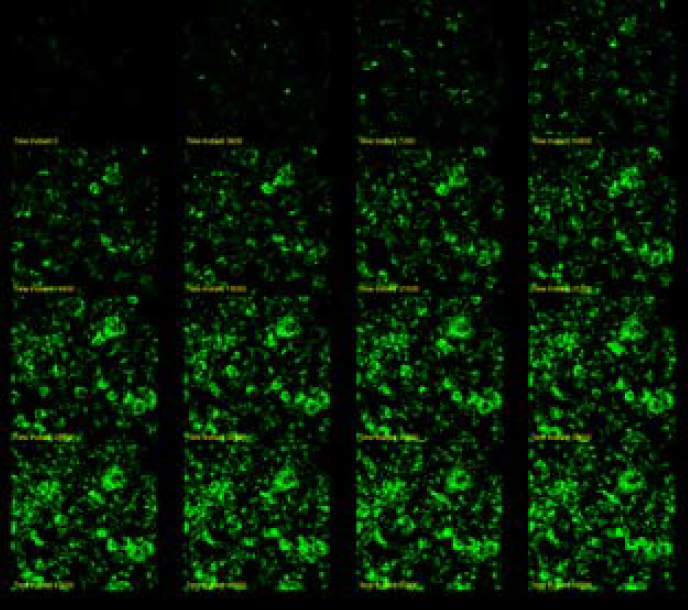
Trastuzumab (anti-HER2) was labeled with pHAb dye. SKBR3 cells were treated with 30nM Trastuzumab-pHAb dye. Image was captured every 60 minutes.
Contact us to see data in this poster: A New Bioluminescent Assay for Quantifying Antibody Internalization in Non-Engineered Cells Using NanoLuc® Technology
Fc-Receptor Characterization
Understanding Fc receptor (FcR) interactions is pivotal for ADC development, directly impacting their mechanism of action and immune system engagement. This characterization is essential for optimizing ADC efficacy through antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), shaping the safety and therapeutic profiles of these advanced treatments.
ADCC and ADCP
ADCC involves immune cells targeting and destroying antibody-coated cells, while ADCP refers to the engulfing and digestion of target cells by phagocytes. Our Fc Effector Activity Bioassays are specifically designed to quantify the potency of these Fc effector functions, providing researchers with essential data to guide ADC development.
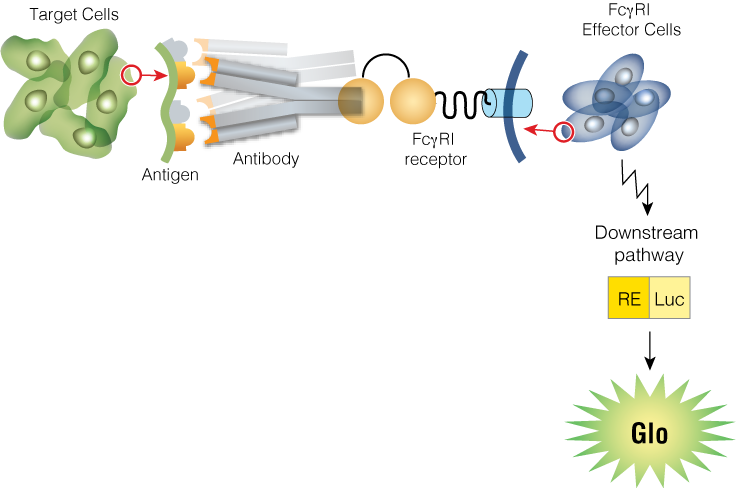
Measurement of ADC Fc Effector Activity using ADCC and ADCP Reporter Bioassays


Serial dilutions of trastuzumab (anti-HER2 monoclonal antibody), trastuzumab emtansine, or trastuzumab deruxtecan were incubated for 6 hours at 37°C with ADCC Bioassay Effector Cells (left panel) or ADCP Bioassay (THP-1) Effector Cells (right panel) and SKOV3 or SKOV3/HER2-knockout target cells, as indicated.
See a complete list of our Fc Effector Activity Bioassays, including a variety of ADCC and ADCP Reporter Bioassays.
Measure Fc Receptor Binding Across Isotypes
Lumit® FcγR Binding Immunoassays are novel homogeneous (no-wash) competition assays to measure the interaction between human Fc receptors and antibodies or Fc fusion proteins.
Lumit® FcRn Assay

Lumit® FcγRIIa (H131) Assay

Lumit® FcγRIIIa (R158) Assay

CDC
Complement-dependent cytotoxicity (CDC) leverages the immune system's ability to destroy tumor cells. The CytoTox-Glo™ Cytotoxicity Assay offers a robust method for quantifying CDC-mediated cell killing, providing valuable insights into the potential of ADCs to engage and activate the complement pathway for targeted cancer cell eradication.

Daudi cells were treated with rituximab in RPMI 1640 + 12.5% human serum (the complement source). After 2hr, CytoTox-Glo™ was used to measure CDC on the Glomax® Discover.
Payload Function
Cytotoxicity
Explore the impact of cytotoxic ADC payloads with our cell viability and apoptosis assays. These tools offer real-time insights into how ADC payloads affect target cell survival and death mechanisms, crucial for optimizing therapeutic design and efficacy. Through dynamic monitoring, ADC researchers can precisely evaluate payload-induced cytotoxic effects and apoptosis, guiding the development of more effective cancer treatments.
See more data in this poster: Accelerating Cell-Based ADC Potency Assay Development with Real-Time Assays



Immunogenic Cell Death
Immunogenic cell death (ICD) is crucial in ADC research as it enhances the immune system's ability to recognize and destroy tumor cells. ADCs can induce ICD, leading to the release of cancer antigens and recruitment of immune cells to the tumor site. This process not only amplifies the direct killing effect of the ADC but also promotes a long-lasting immune response against the tumor, making ICD an important mechanism for improving the efficacy of ADC therapies. Our specialized assays allow you to explore the critical role of ICD in your ADC research. Learn more in this article: Immunogenic Cell Death: Methods to Measure Damage Associated Molecular Patterns (DAMPS).
Lumit® Immunoassays allow you to measure the crucial cytokines signaling ICD. These assays provide precise, reliable quantification of key pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and IFN-γ, essential for evaluating the immune system's response to ADC therapies. Learn more about our Lumit® Cytokine Immunoassays.

Degraders
We offer a suite of specialized tools for analyzing the function of ADC payloads that are degraders or PROTACs. These tools allow you to meticulously evaluate the efficacy and mechanism of action of degrader payloads, facilitating a comprehensive understanding of protein degradation pathways. Visit our Targeted Protein Degradation web page for a deep dive into degrader analysis tools.
Oligos
We offer solutions for exploring the dynamics and therapeutic impact of ADC payloads involving oligonucleotides or RNA-targeting strategies. These tools enable detailed analyses of RNA-targeting payload actions and their implications for drug development. Explore our Targeting RNA web page for details.
Specificity
Analyzing the bystander killing effect and specificity of your ADC molecule is crucial for optimizing therapeutic efficacy and minimizing off-target effects. This evaluation ensures that the ADC not only targets and eliminates diseased cells but also assesses its impact on surrounding healthy tissues. Our HiBiT Target Cell Killing Biossay allows you to look at death of a specific cell type within a mixed culture. Explore our list of available cell lines.

See data in this poster: Novel Bioluminescent Tools for the Assessment of ADC Fc Function and Bystander Killing
Services
- Custom Assay Development: Tailor-made assays for specific analysis needs.
- Screening: Identify and optimize lead candidates efficiently.
- Mechanism of Action Studies: Detailed insights into how your ADC works.
- Real-Time Cell Death Analysis: Monitor ADC efficacy in live cells.
- Protein Degradation and RNA-Targeting Analysis: Explore the intricacies of your payload's action.
Our team is equipped to support your unique research requirements, driving your ADC development forward.





