Molecular Characterization for Cell Therapy
Molecular characterization, such as DNA sequence verification, is crucial in the development and manufacturing of cell therapies. It allows for the identification of genetic modifications, verification of cell identity, and evaluation of potential safety risks, ultimately improving the efficacy and safety of these therapies. We provide a comprehensive range of user-friendly nucleic acid extraction and analysis solutions, which are widely used in cell therapy development and manufacturing processes.
Need an automated solution for nucleic acid extraction? Learn how our Maxwell® Instruments can be used for cell therapy applications.
Do My Cells Contain the Correct CAR Sequence?
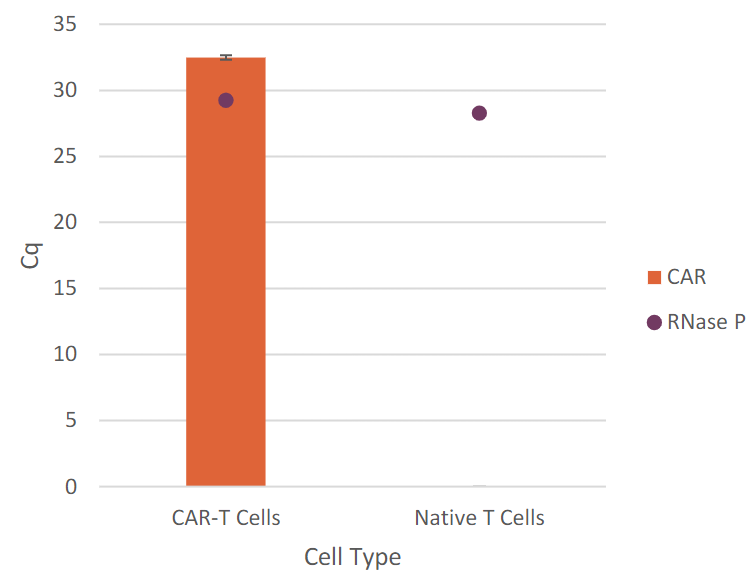
CAR-T cells are genetically engineered T cells designed to target and destroy cancer cells by expressing a chimeric antigen receptor (CAR) specific to tumor antigens. It is essential to confirm that the CAR-T products express the correct CAR, ensuring targeted efficacy and patient safety. As part of CAR-T characterization and quality control (QC) assays, CAR transgene copy number and sequence confirmation are often done by qPCR and Sanger sequencing.
Plasmid DNA sequence confirmation is a critical step in the development of CAR-T cell therapy. Our workflow for plasmid DNA Sanger Sequencing can ensure integrity of your therapy, enhancing both efficacy and safety.
Automated Workflow for CAR Sequence Confirmation and Copy Number Determination in CAR-T Cells
Serially dilute CAR-positive transduced T-cells in native T-cells
Automated DNA Purification from CAR-T Cells
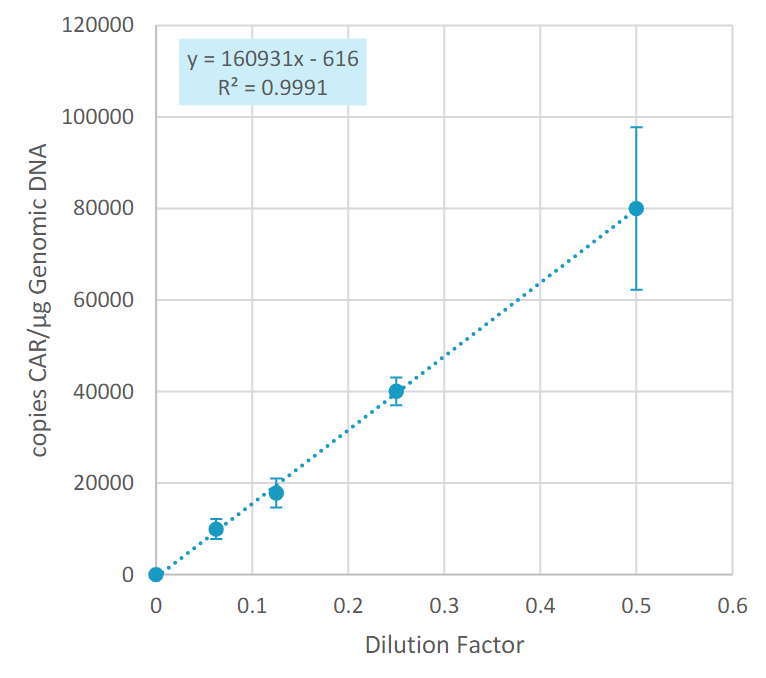
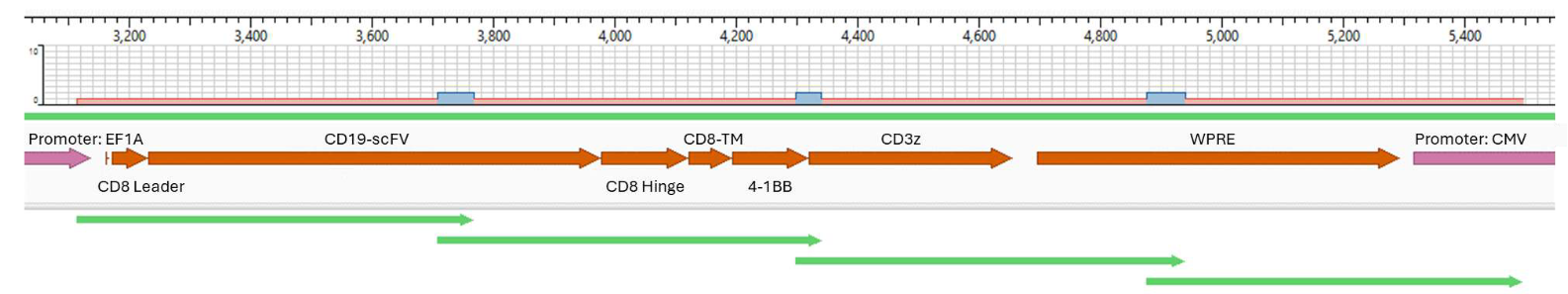
Sanger sequencing the CAR construct in CAR-T cells. DNA was purified from 50,000 CD19-CAR-T cells. Amplicons were prepared across 4 overlapping sections of the construct and were sequenced using the ProDye® Terminator Sequencing System and the Spectrum Compact CE System. Map of the assembled contig and coverage across the construct is shown.
See the full protocol and data in this application note: Automated DNA Purification from CAR-T Cells
See selected citations on using Maxwell® Kits and Instruments to extract DNA/RNA for molecular characterization:
| Product Used | Citation |
|---|---|
| Maxwell® RSC miRNA Plasma and Serum Kit and Maxwell® RSC Instrument were used to purify circulating miRNAs from plasma collected from patients who underwent CAR-T cell infusion. | De Matteis, S. et al. (2023) Peripheral blood cellular profile at pre-lymphodepletion is associated with CD19-targeted CAR-T cell-associated neurotoxicity. Front. Immunol. 13, 2022. |
| Maxwell® RSC simplyRNA Blood Kit and a Maxwell® RSC Instrument were used to extract mRNA from tumor cells and fibroblasts before RT-qPCR. | Wagner, J. et al. (2021) Antitumor effects of CAR T cells redirected to the EDB splice variant of fibronectin. Cancer Immunol. Res. 9(3), 279–290. |
| Maxwell® RSC Whole Blood DNA Kit was used to purify DNA from peripheral blood samples before PCR and dPCR for detecting CAR-T cells in patients. | De la Iglesia-San Sabastian, I. et al. (2024) Digital PCR improves sensitivity and quantification in monitoring CAR-T cells in B cell lymphoma patients. Transplant. Cell. Ther. 30(3), 306.e1. |
| Maxwell® RSC simplyRNA Blood Kit and Maxwell® RSC Instrument were used to extract mRNA from single cell suspensions of cultured cell lines and patient-derived xenograft model tissue samples. | Shaw, T.I. et al. (2024) Discovery of immunotherapy targets for pediatric solid and brain tumors by exon-level expression. Nat. Commun. 15, 3732. |
| Maxwell® RSC RNA FFPE Kit was used to isolate RNA from FFPE tumor samples to identify tumor-associated antigen specific T-cell responses. | Thelen, M. et al. (2022) Immune responses against shared antigens are common in esophago-gastric cancer and can be enhanced using CD40-activated B cells. J. Immunother. Cancer 10(12), e005200. |
Do My Cells Share the Same Identity as the Parental Cells?
Cell identity testing is crucial to ensure the correct cells are used or manufactured, which is essential for both the safety and efficacy of the therapy. Short Tandem Repeat (STR) sequences are unique to each donor. Therefore, STR profiles can be used as an accurate tool to identify each donor cells.
Our workflow using the GenePrint® 24 System combined with the Spectrum Compact CE System can provide highly informative STR analysis for cells used in CAR-T cell development and manufacturing, such as assessing or confirming cell identity.
CAR-T Donor Authentication by DNA Fingerprinting Workflow
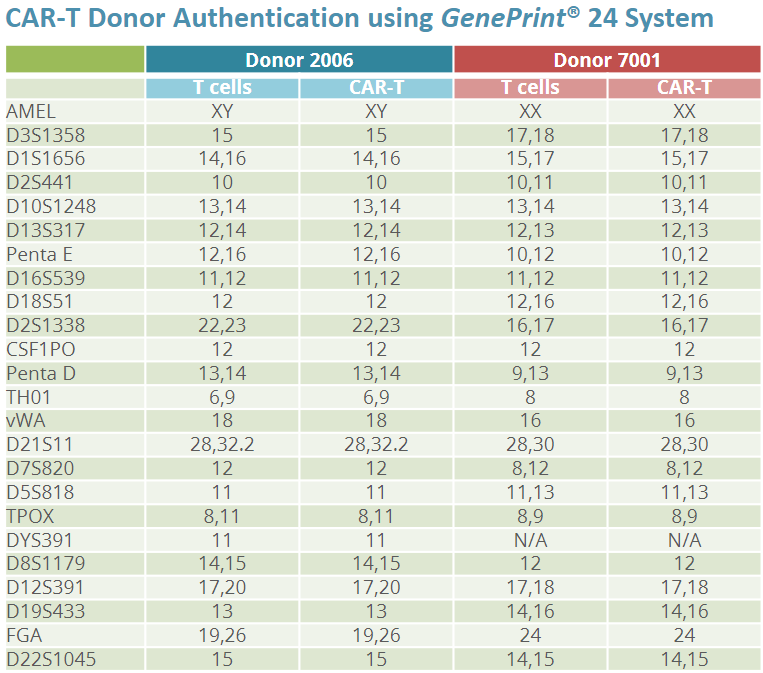
Unstimulated T Cells and finished CAR-T product were spotted on FTA cards using direct amplification of the GenePrint® 24 System and analyzed on a Spectrum Compact CE System, shows 100% concordance of allele calls within donors.
Are There Mycoplasma or Other Contaminants in My Cells?
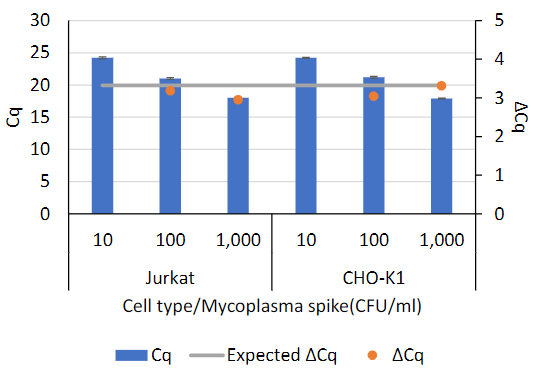
Detecting mycoplasma in cell therapy products is crucial because contamination can compromise the safety and efficacy of these therapies, and stringent regulatory requirements mandate rigorous testing to ensure product purity and patient safety.
You can purify and detect mycoplasma as low as 10CFU/ml in tissue cell culture and cell culture supernatant with an automated workflow using Maxwell® RSC and RSC 48 Instruments.
Mycoplasma Detection Workflow

Aliquot cell culture or cell culture supernatant