Potency and Functional Assays for Cell Therapy
Potency and functional assays are fundamental to the development of cell therapies, as they provide critical insights into the therapeutic potential and consistency of the cell products. Potency assays measure the biological activity of the cells, ensuring they can achieve the desired therapeutic effect, while functional assays assess the specific capabilities of the cells, such as their ability to differentiate, secrete factors, or target diseased cells. Together, these assays are essential for validating the efficacy, safety, and reliability of cell therapies. Learn about important questions to ask regarding the potency and functionality of your cells during the development of cell therapies and methods that can help answer them.
Join this webinar to learn about a new target cell killing-based approach for measuring CAR-T potency.
Do My Cells Produce the Expected Proteins or Cytokines?
Lumit® Technology provides a highly sensitive, luminescence-based method for detecting whether your cells are producing the expected proteins or secreting the desired cytokines. With its simple, rapid "no-wash" protocol, these assays offer a faster and more reliable alternative to ELISAs. They enable direct detection and quantification of biomolecules in the cell culture medium, delivering real-time insights into cellular function and protein expression. These assays are especially useful for verifying the functionality of cell therapies, as it allows precise measurement of target protein or cytokine levels to ensure cells are performing as intended.
CAR-T Cells
Lumit® Cytokine Immunoassays are specifically designed to detect cytokine production from activated, engineered immune cells, such as CAR-T or CAR-NK cells. These assays can be run directly on cells, eliminating the need for media transfer, dilution, or wash steps, which are common sources of error. With a broad linear range, Lumit® Cytokine Immunoassays are capable of covering expected concentration ranges from most cell models.
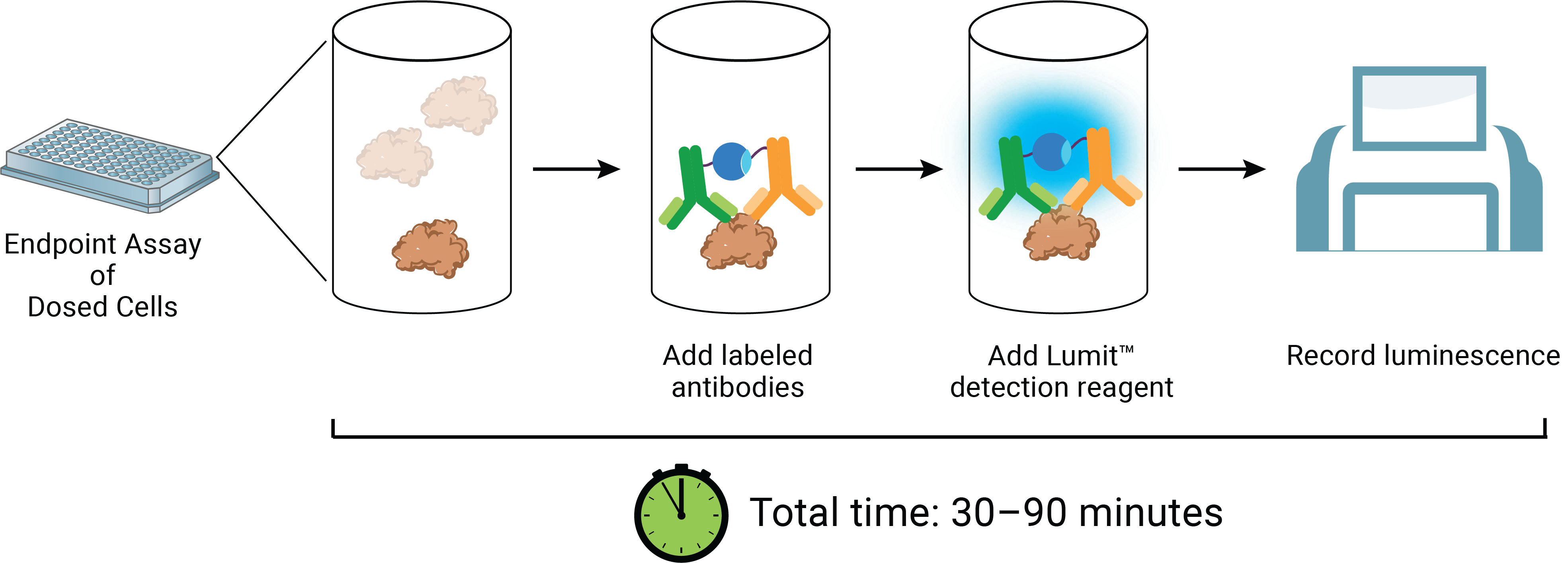

Left Panel: Simple, no-wash Lumit® Immunoassay workflow. Right Panel: Robust measurement of CAR-T cytokine secretion. T cells transduced with CAR-19 lentivirus were combined with Ramos target cells at different E:T Ratios. After 24 hours incubation, Lumit® IFN-γ (Human) Immunoassay was used to quantify IFN-γ secretion.
iPSC-derived Cells
Induced pluripotent stem cells (iPSCs)-derived cell therapy is another promising approach to treating a range of conditions, including neurodegenerative diseases (e.g., Parkinson’s), heart disease, diabetes and cancer. iPSCs can be differentiated into specific cell types, such as neurons, cardiomyocytes, or insulin-producing cells, which can then be used to replace damaged or diseased tissues. Functional assays are critical for evaluating the functionality and quality of iPSC-derived cells, ensuring that they effectively mimic natural cells and are capable of performing key tasks (e.g., insulin secretion in iPSC-derived pancreatic beta cells).
Lumit® Insulin and Glucagon Immunoassays are homogeneous, bioluminescent ELISA alternatives to measure insulin and glucagon from pancreatic islets or iPSC-derived pancreatic cells in culture. The assays offer a fast, easy, no-wash protocol and are ideal for quantifying hormone secretion from islet cells.
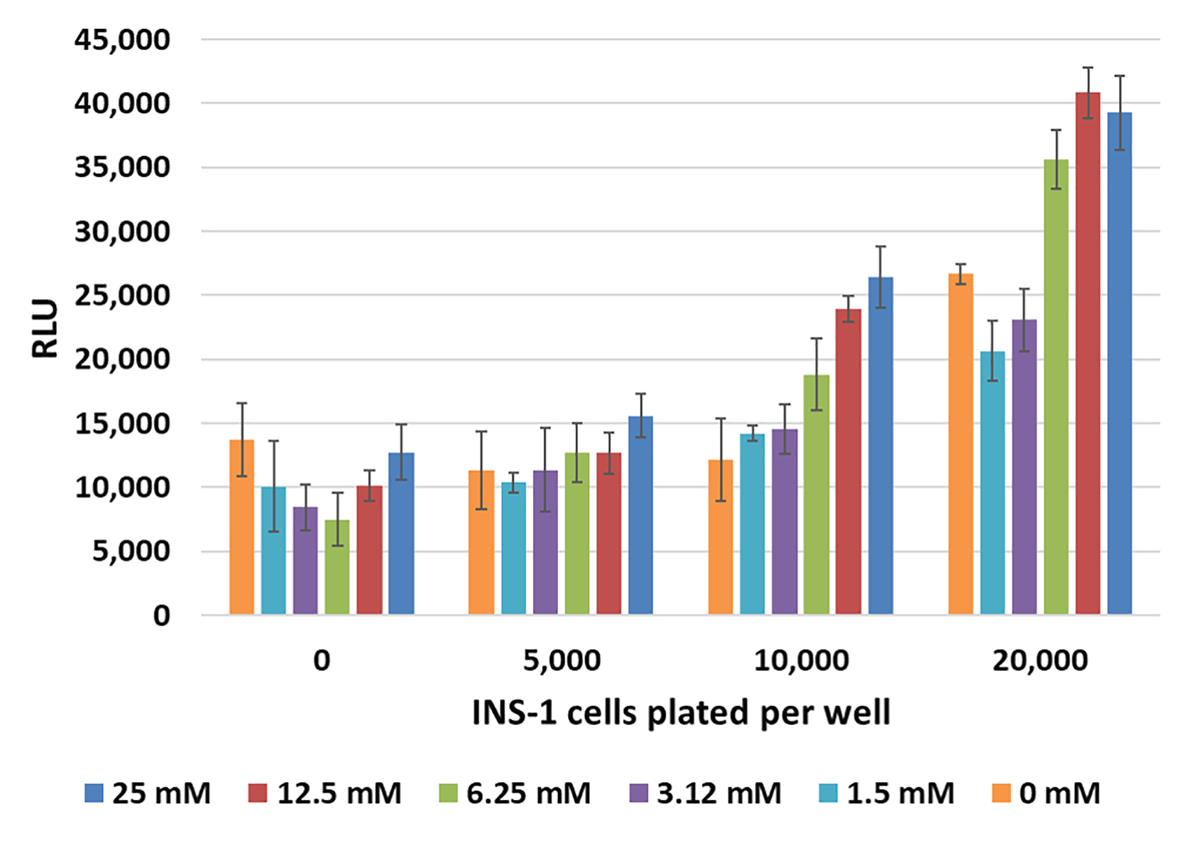
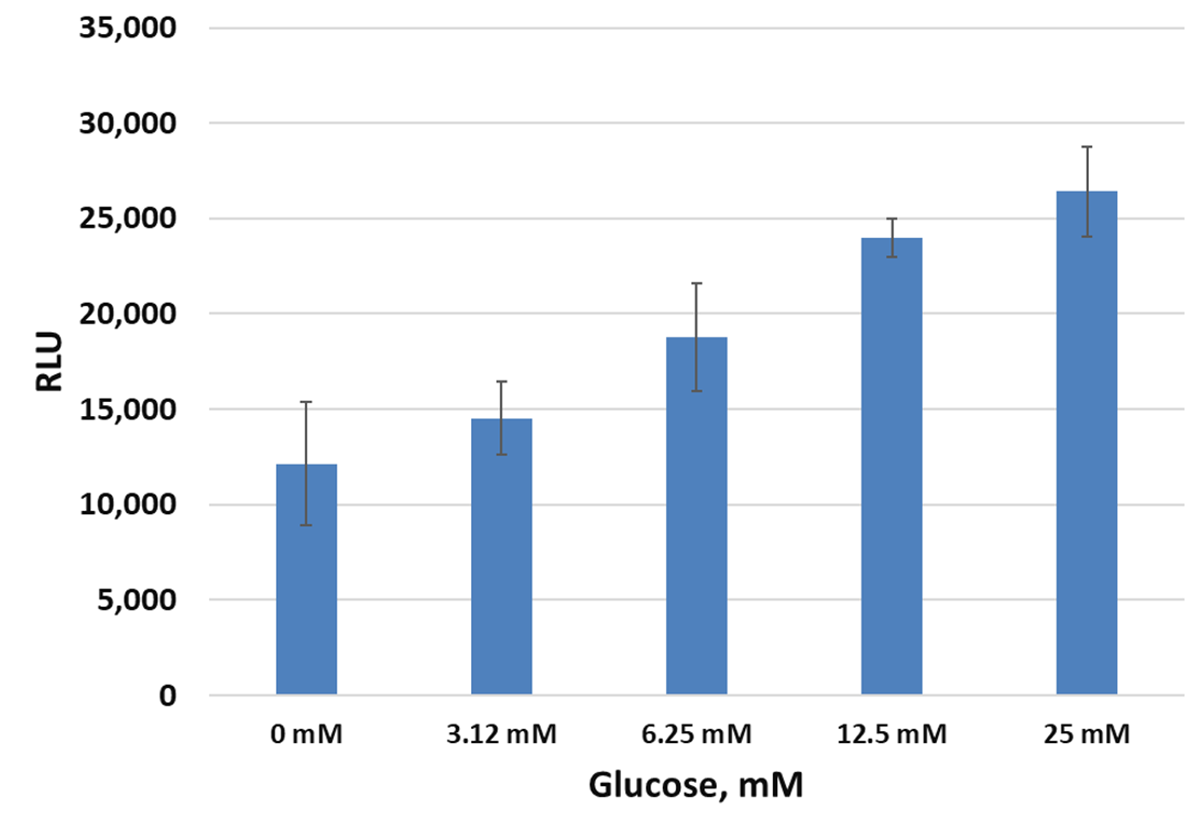
Monitoring cellular insulin secretion response to glucose with Lumit® Immunoassays. Left Panel: INS-1 cells plated in duplicate at varying cells/well in 96-well plates were used for a glucose-stimulated insulin secretion experiment. After 60 minutes, 10µl of supernatant was removed and assayed with the Lumit® Insulin Immunoassay in 384-well plates. Error bars are +/–1 s.d. Right Panel: The data from the same experiment using 10,000 cells/well is shown, demonstrating increasing release of insulin with increasing concentration of glucose.
How Potent Are My CAR-T Cells in Target Cell Killing?
Potency assays should be designed to specifically measure the intended biological effect of a cell therapy. In the case of immune cell therapy (e.g., CAR-T, TCR-T, TILs and CAR-NK), target cell killing assays are crucial in providing essential data on the efficacy, safety, and functionality of the cells during the preclinical development and manufacturing processes, helping to refine therapy for clinical use.
Many existing viability assays are not target cell-specific, posing challenges in accurately measuring the therapeutic effect. Other common cell killing assays using fluorescent dyes and flow cytometry suffer from long process, high donor variability, handling time, low sensitivity/high background and complicated analysis with no standardization.
Gain of Signal Assays
The MOA-based HiBiT Target Cell Killing (TCK) Bioassays are simple to run and enables highly sensitive and specific measurement of target cell killing by the effector cells. Unlike the lost of luminescence signal upon the killing of tumor cells expressing Firefly luciferase, the HiBiT TCK Bioassays produce a bright luminescence signal upon cell lysis by the effector immune cells.


Left Panel: Principle of the HiBiT TCK Bioassay. Cytotoxic mAbs and/or effector cells are incubated with target cells expressing a HiBiT fusion protein. Upon killing of the target cell, the HiBiT fusion protein is released and binds extracellular LgBiT to create a functional NanoBiT® Luciferase enzyme. Luminescence is measured using a luminometer. Right Panel: Measuring CAR-T cell-mediated cytotoxicity with HiBiT TCK Bioassays. H929 Cells (HT-HiBiT) were incubated with serially diluted human BCMA CAR-T cells at the indicated E:T ratios. After a 4-, 24-, 48- or 72-hour induction, Bio-Glo-NB™ TCK Reagent was added and luminescence quantified using the GloMax® Discover System. HT = HaloTag, the fusion partner for the HiBiT tag.
Loss of Signal Assays
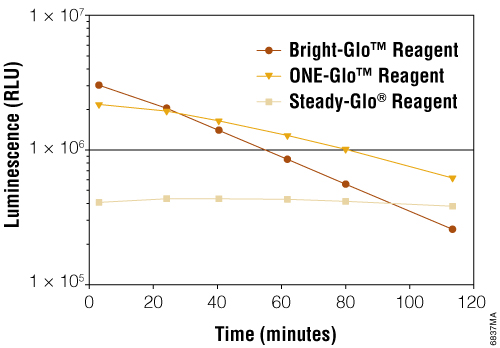
The cytotoxic effect of CAR-T or other immune cells toward tumor cells are also often determined by co-culturing of the immune cells with tumor cells engineered to express a luciferase, such as Firefly luciferase. Our Bright-Glo™ (Foukle et al., 2024; Kim et al., 2023; Rodriguez-Marquez et al., 2022) and One-Glo™ Luciferase Assay Systems (Panowski et al, 2020) are highly sensitive, robust, homogeneous assays for detecting firefly luciferase reporter gene expression in mammalian cells. The decrease of the luminescence signal from the engineered tumor cells indicates the cytotoxicity effect of the effector immune cells.
How do I Screen and Validate New TCR/CARs?
During the discovery and development of new antigen specific T cell receptors (TCRs) or CARs, our bioluminescent luciferase reporter T Cell Activation Bioassays can be used to monitor or validate TCR or CAR function (Nishimura et al., 2024; Uchibori et al., 2019; Jahan et al., 2023)
Lentivirus vectors used in CAR-T manufacturing require additional measures of potency beyond transgene expression of the viral vector in CAR-T cells. Our MoA-based T Cell Activation Bioassays measure CAR:target cell engagement for T cell redirecting lentiviral vectors, providing a stability indicating, quantitative luminescent readout that can fit in your CAR-T workflow.
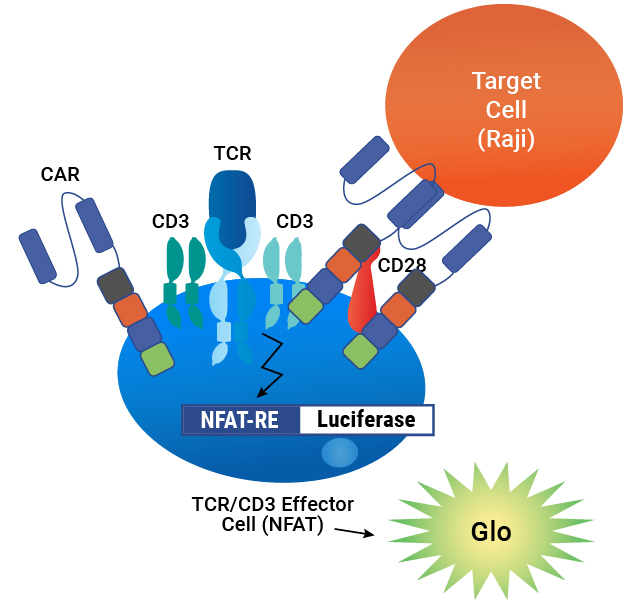
Assay principle. CAR is expressed on the surface of TCR/CD3 Effector Cells containing an NFAT-Luciferase reporter. When the CAR is engaged by an antigen on a target cell, luminescence is induced in proportion to the signaling.

The T Cell Activation Bioassay (NFAT and IL-2) each consists of a genetically engineered cell line, TCR/CD3 Effector Cells NFAT (Panel A) or IL-2 (Panel B). When engaged with either an anti-TCR/CD3 stimulus alone or an anti-TCR/CD3 and an anti-CD28 stimulus, receptor-mediated signaling induces luminescence.
Lentivirus Bioassay is Specific


Lentivirus Bioassay Is Stability Indicating

| Heat Treatment Time (hours) |
EC50 |
% Inhibition of Max |
|---|---|---|
| 0 |
0.81 | Reference |
| 2 |
1.0 |
26% |
| 6 |
2.4 |
18% |
| 24 | 2.5 |
47% |
| 48 | 5.0 |
67% |
Forced degradation samples of CAR-19 lentivirus were prepared by incubating at 37°C for 2–48 hours, then analyzed using TCR/CD3 Effector Cells and Raji Target Cells. The assay demonstrates a loss of lentiviral potency with heat treatment, as measured by an increase in EC50 and a decrease in the maximum response.
Our T Cell Activation Bioassay (TCRαβ-KO) is designed for those developing TCR-T cell therapies and need to measure the potency and specificity of candidate TCRs. These assays lack expression of endogenous TCRαβ chains, which eliminates endogenous alpha and beta chain cross-pairing with transgenic TCRs, providing an improved assay window.

Principle of the T Cell Activation Bioassay (TCRαβ-KO). Without expressing a transgenic TCR, the TCRαβ-KO Cells are not activated by peptide or MHC, resulting in low light output. TCRαβ-KO Cells transfected or transduced to express a TCR are activated by APCs and cognate antigen, inducing TCR pathway-activated luminescence.


Activity of transgenic TCRs measured using the T Cell Activation Bioassay (TCRαβ-KO, CD4+). Panel A. TCRαβ-KO (CD4+) Cells were transfected with a Positive Control TCR Plasmid (or mock transfected) and then co-cultured with MHCII APC Cells (HLA-DR+ cells) and increasing concentrations of the Positive Control Peptide. Panel B. TCR-mediated T cell activation was measured using the T Cell Activation Bioassay (endogenous TCR, CD4+) and the T Cell Activation Bioassay (TCRαβ-KO, CD4+). The T Cell Activation Bioassay cells were co-cultured with HLA-DR-positive cells and increasing amounts of cognate peptide. The modified T cells are activated once they bind antigens on tumor cells, as indicated by luminescence. The data demonstrate that the T Cell Activation Bioassay can be used to screen transgenic TCRs used for T cell immunotherapy.
Are My Cells Functional in Animal Models?
In vivo bioluminescence imaging is widely used for monitoring the cell fate, distribution, efficacy and/or potential risks of cell therapies in live animals. Luciferase-based reporters can achieve high signal-to-background ratios due to a lack of autofluorescence, offering sensitive, noninvasive imaging of tissue cells with lower detection limits.
Firefly luciferase (FLuc) and NanoLuc® luciferase (NLuc) can be used together for dual-imaging as there is no substrate cross-reactivity between the two enzymes. Multiplexing using these bioluminescent reporters have been demonstrated to track the localization and killing of tumor target cells by CAR-T cells (Su et al., 2020) and CAR-NK cells (Shalaby et al., 2024).
Luminescent Reporters and Substrates
| Reporter | Substrate |
|---|---|
| Firefly luciferase (FLuc) | VivoGlo™ Luciferin, In Vivo Grade |
| NanoLuc (NLuc) | Nano-Glo® Fluorofurimazine In Vivo Substrate (FFz) |
| Renilla (RLuc) | ViviRen™ In Vivo Renilla Luciferase Substrate |
Can't find a bioassay that fits your exact needs?
Our Tailored R&D Solutions team can help development a custom bioassay just for you.