Changes in Energy Metabolism
To support growth, cancer cells reprogram their energy metabolism to use glycolysis as the main source of energy production–even in the presence of oxygen–in a process known as “aerobic glycolysis.” This change from a resting to a proliferative cell metabolism is characterized by increased glucose and glutamine uptake with increased lactate secretion and oxygen consumption. Within the tumor microenvironment, changes in metabolite levels often result in altered immunometabolic pathways of T cells and other immune cells. Tumor cells also have increased levels of oxidative stress, which is caused by accumulation of reactive oxygen species (ROS).
Glycolysis and Glutaminolysis
The two major metabolic pathways in cancer are glycolysis and glutaminolysis. Glycolysis can be monitored using live-cell analysis by measuring glucose consumption and lactate secretion in tumors, while glutaminolysis can be monitored by measuring glutamine and glutamate levels. Glutamine and glutamate respond to the increased ROS production of a proliferating cell, as well as supply intermediates for the TCA cycle.

Metabolism Assays Q&A
In this webinar, our experts introduce and compare methods for monitoring metabolites (e.g., glucose, lactate, glutamate, glutamine) in cancer and immune cells. They answer submitted questions, such as "What is the difference between glucose detection and glucose uptake assays?" and "How can I normalize metabolite levels to cell number?"
Glucose Uptake Assays
What are the pros and cons of various methods used to measure glucose uptake? Read this article to find out.
Cancer's Need for Metabolites
This blog explores how cancer's need for metabolites offers potential targets for halting tumor progression.
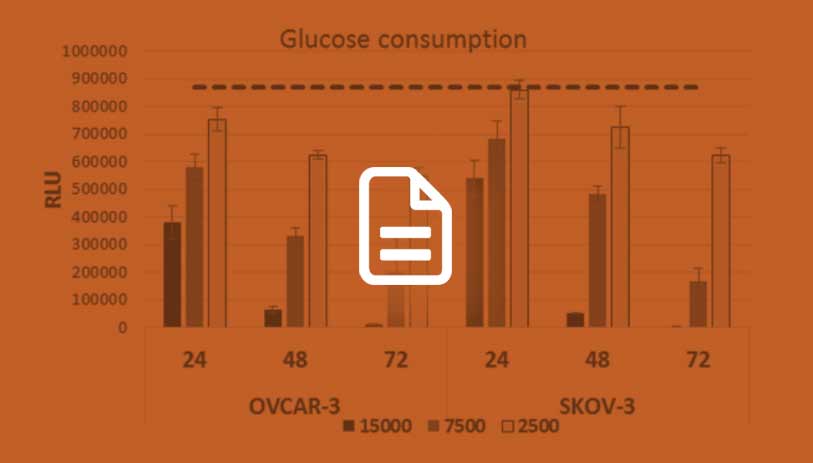
High-Throughput Metabolism Assays
Download this article to learn about bioluminescent assays suitable for high-throughput analysis of glycolysis and glutaminolysis.
Interested in monitoring glycolysis or glutaminolysis?
Oxidative Stress
Tumor cells generate high levels of reactive oxygen species (ROS) due to increased metabolic activity and oncogenic stimulations. ROS, such as H2O2, promote cell proliferation and genomic instability, leading to tumor progression. However, excessive ROS can suppress tumor growth and induce cell death. In order to counterbalance ROS levels, tumors also produce antioxidants, such as glutathione (GSH), which requires various co-factors (NAD+, NADH, NADP+ and NADPH).
Detection of ROS
Read this article to learn about a fast and sensitive bioluminescent assay that measures the level of H2O2 directly in cell culture.

Glutathione Quantification
Download this article to learn about a homogeneous bioluminescent assay used to monitor glutathione levels in human leukemic cells after drug treatment.
NAD(P)/NAD(P)H Quantification
View this webinar to learn about bioluminescent assays for rapid and sensitive measurement of redox-defining co-factors NAD(P)/NAD(P)H.
Interested in monitoring oxidative stress?
Immunometabolism
Immunometabolism is an emerging field that studies the interplay between immunology and metabolism. Within the tumor microenvironment, metabolite levels often change due to local nutrient depletion or production of metabolic waste. As a result, the intracellular metabolic pathways of T cells and other immune cells can be activated or suppressed. Monitoring metabolite levels is an effective way to study immune cell activation in tumors.

Monitoring T Cell Activation
View this webinar to learn about monitoring changes in metabolite levels over time during T cell activation and growth of cancer cell lines.

Monitoring T Cell Activity with Bioluminescent Assays
This poster provides an example of studying T cell activation by monitoring glycolysis and increased lactate secretion over time.

A Guide to Immunometabolism
This review paper is a refresher course of six main cellular metabolic pathways and their possible roles in immunity.
Need a metabolic assay for immunometabolism research?