NLRP3 Inflammasome Activation
The inflammasome is a large protein complex that assembles in response to pathogens and other stressors. It activates the inflammation response and induces pyroptosis, a form of cell death. Dysregulation of the inflammasome has been implicated in the pathogenesis of various chronic inflammatory, autoinflammatory and autoimmune disease.
Promega offers several assays and technologies to monitor inflammasome activation, including quantifying target engagement of NLRP3 inflammasome NACHT domain inhibitors in live cells, detecting caspase-1 activation and quantifying IL-1β release. In addition, we provide an assay to detect extracellular ATP, a known inflammasome trigger.
Interested in learning more about monitoring inflammasome activation?
Learn how the NanoBRET® TE NLRP3 assay provides insight into NLRP3 activation.
The NLRP3 inflammasome detects stress signals, like extracellular ATP released by damaged cells. These signals activate the inflammasome and recruit caspase-1 to the inflammasome complex. Caspase-1 then cleaves and activates the proinflammatory cytokine, IL-1β. NLRP3 inhibitors, such as MCC950, show promising results as therapeutic agents to target the inflammation response.

Learn more about inflammation assays and measuring cell health, or view a free webinar.
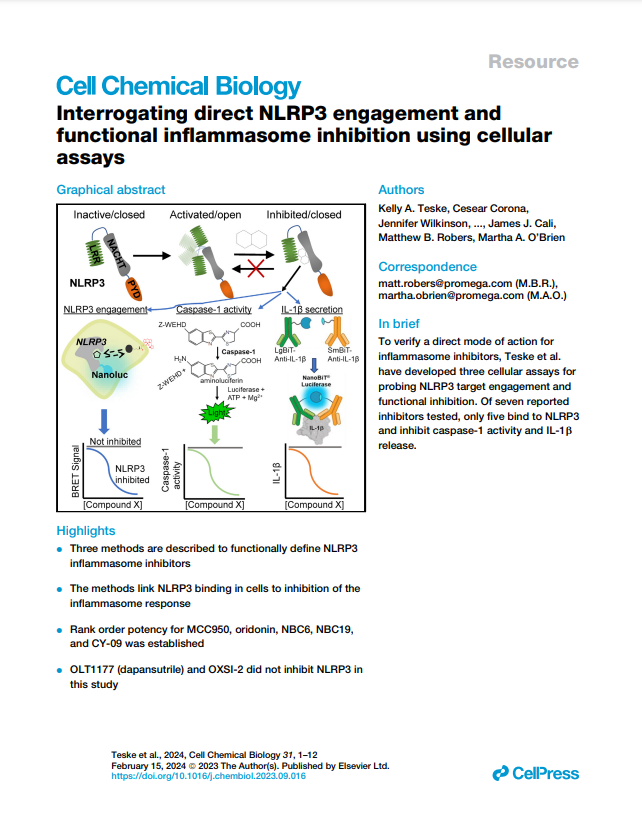
Featured Publication
This peer-reviewed study "Interrogating direct NLRP3 engagement and functional inflammasome inhibition using cellular assays" examines the direct engagement and functionality of NLRP3 pathway activity in cells using several mechanistic assays, including the Lumit® IL-1β Immunoassay and Caspase-Glo 1 Inflammasome Assay.
Learn more about assays and technology to investigate NLRP3 pathway antagonism in this video presentation delivered at the Inflammasome Therapeutic Summit 2021.

Caspase-1 Activation
The activated inflammasome recruits caspase-1 and activates it through conversion of procaspase-1 zymogen into catalytically active caspase-1. Caspase-1 activation results in the processing and release of cytokines IL-1β and IL-18, as well as pyroptosis, an immunogenic form of cell death.
The Caspase-Glo® 1 Assay is sensitive enough to measure caspase-1 activity directly in cells or in medium in multiwell plates. Caspase cleavage of Z-WEHD-aminoluciferin releases aminoluciferin, resulting in luciferase activity and generation of light by a proprietary, thermostable recombinant luciferase.


Caspase-1 activity detected in peripheral blood mononuclear cells (PBMCs). PBMCs from four-donor pools (BioIVT) were plated in 96-well plates in RPMI-1640 + 10% FBS at 7.5 x 105 cells/ml and incubated with a titration of LPS overnight followed by a 2 hour treatment with 20 µM nigericin or vehicle. Caspase-1 activity was monitored with the Caspase-Glo® 1 Inflammasome Assay. Caspase-Glo® 1 Reagent +/- YVAD-CHO was added and luminescence recorded after 1.25 hours.

Caspase-1 activity detected in J774A.1 macrophages. Mouse J774A.1 cells at 5 x 105 cells/ml in DMEM + 10% FBS in 96-well plates were LPS-primed or left unprimed, then treated with a time course of nigericin (20 µM). Caspase-1 activity was monitored with the Caspase-Glo® 1 Inflammasome Assay. Caspase-Glo® 1 Reagent +/- YVAD-CHO was added and luminescence recorded after 1.5 hours. Modified from O'Brien et al. (2017) J. Immunol. Meth. 447, 1–13.
Learn more about caspase-1 activation assays in this publication
or visit the product page.
Cytokine Release
Lumit® Immunoassays quantitatively measure cytokines released in cell culture samples using a simple, no-wash protocol. The sensitive assay generates a luminescent signal that can be read on any standard plate-reading luminometer.
- Experiment completed in less than 70 minutes
- Use directly on cells in culture or culture medium transferred to a separate assay plate

Quantify IL-18 Release
The Lumit® Active IL-18 Immunoassay uses one primary antibody to IL-18 labeled with the LgBiT subunit of NanoBiT® luciferase in combination with the IL-18 Binding Protein, labeled with the SmBiT subunit of NanoBiT® Luciferase. In the presence of cleaved, mature IL-18, the NanoBiT® subunits are brought together to form an active luciferase enzyme. Addition of the optimized luciferase substrate generates a bright luminescent signal proportional to active IL-18 levels.


Active IL-18 release quantified from peripheral blood mononuclear cells (PBMC). Human PBMC (4-donor pools, BioIVT) were added to 96-well plates at 100,000/well in RPMI-1640 + 10% heat-inactivated FBS with the IL-18bp-SmBiT. MCC950 (4µM) or vehicle was added to the PBMC followed by LPS (500ng/ml) or vehicle. The next day, nigericin (10µM) or vehicle was added and incubated for 2 hours. IL-18 release was measured with the Lumit® Active IL-18 (Human) Immunoassay directly in the cell wells. LPS activated the alternative NLRP3 pathway and LPS + nigericin stimulated the canonical NLRP3 inflammasome pathway.
Quantify IL-1β Release

IL-1β release quantified from PBMCs. PBMCs from four-donor pools (BioIVT) were plated in RPMI-1640 + 10% FBS at 5.5 x 105 cells/ml in 96-well plates and 1.65 x 105 cells/ml in 384-well plates, then treated with a titration of LPS overnight. The next day, IL-1β release was quantified with the Lumit® IL-1β (Human) Immunoassay directly in the cell wells.

IL-1β release quantified from J774A.1 macrophages. Mouse J774A.1 cells at 5 x 105 cells/ml in DMEM + 10% FBS in 96-well plates were LPS-primed (500ng/ml) or left unprimed overnight, then treated the next day with a time course of nigericin (20µM). IL-1β release was quantified with the Lumit® IL-1β (Human) Immunoassay directly in the cell wells.
Want to measure other cytokines? See all available Lumit® Immunoassays for cytokine detection.
NLRP3 Target Engagement (TE)
NLRP3 is expressed mostly in macrophages and is a part of the caspase-1 activating complex known as the NLRP3 inflammasome. The NLRP3 NACHT domain is an attractive target for potential inflammation inhibitors, such the diarylsulfonylurea compound MCC-950. We have developed a NanoBRET® probe that can quantify engagement of NACHT domain inhibitors in live cells.

Using a NanoBRET® probe to detect the NLRP3 activation state in live cells.
With the NanoBRET® Target Engagement Assay, you can:
- Quantitate compound affinity (how tightly it binds to a protein) and target protein occupancy (how much compound binds to a protein) in live cells.
- Assess how long a compound binds to the target protein (its residence time) under physiological conditions.
- Scale the simple multiwell assay for your research.
- Generate high‐quality data with low error rate and high reproducibility.


Determination of specific NanoBRET® signal and tracer affinity in live cells. HEK293 cells were transiently transfected with NLRP3/Nluc fusion proteins. Cells were treated with serially diluted NLRP3 NanoBRET® tracer, 24 hours after transfection, in the presence or absence of a vast molar excess of unlabeled control ligand. After 2 hours of equilibration, NanoBRET® Target Engagement substrate/inhibitor solution was added and BRET was recorded on a GloMax® Discover Plate Reader.

Measuring unlabeled compound engagement to NLRP3 in live cells. HEK293 cells were transiently transfected with NLRP3/Nluc fusion proteins. Cells were treated with serially diluted NLRP3 NanoBRET® tracer, 24 hours after transfection, in the presence of serially diluted MCC950. After 2 hours of equilibration, NanoBRET® Target Engagement substrate/inhibitor solution was added and BRET was recorded on a GloMax® Discover Plate Reader.
Contact us to request early access to the NLRP3 Target Engagement Assay.
Inflammasome Pathway
The inflammasome pathway is a crucial component of the innate immune system, the body’s first line of defense against pathogens and harmful substances. The broader inflammation pathway is shown below, highlighting how the inflammasome pathway integrates within it.

Created with Biorender.com
Mechanism and Role of Inflammasomes in Innate Immunity
Inflammasomes are multi-protein complexes found within immune cells that play a crucial role in detecting harmful microorganisms and stress signals, thereby triggering an inflammatory response. These inflammasomes are activated by specific intracellular danger signals, which include pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Central to this activation process are pattern recognition receptors (PRRs), such as NLRP3, which are responsible for recognizing these danger signals.
Once the inflammasome is activated, it leads to the cleavage and maturation of pro-inflammatory cytokines, such as IL-1β and IL-18, which are essential in driving the body’s immune response. Furthermore, inflammasome activation also induces pyroptosis, a form of programmed cell death, which plays a role in controlling infections and modulating the inflammation process. This dual function of inflammasomes in cytokine production and cell death highlights their significance in the immune response.
Inflammatory Diseases and Pathogenesis
The innate immune system is a fast-acting defense mechanism against infections, with inflammation serving as a protective response. However, when this process is not properly regulated, it can lead to chronic inflammatory diseases. Dysregulated inflammasome activity is implicated in the pathogenesis of various chronic inflammatory diseases, including rheumatoid arthritis, atherosclerosis, and neurodegenerative conditions like Alzheimer’s. Some viruses and bacteria have evolved mechanisms to evade or manipulate inflammasome signaling, leading to enhanced virulence. As the role of inflammation in these diseases gains more focus, it becomes increasingly more valuable to understand the inflammasome and develop tools to measure its activation.