Custom Assay Development Services
Our custom assay services can help to accelerate your drug discovery and development workflow. These comprehensive services are built around award-winning bioluminescent technology for sensitive, high-throughput and biologically relevant results.
- Customized Support: Benefit from tailored assay development and close collaboration with our expert team.
- Proven Platforms: Leverage state-of-the-art technologies, including bioluminescent reporters, NanoBRET®, NanoBiT®, and Lumit® to support diverse research and therapeutic areas.
- Broad Expertise: Trust our experience in supporting complex workflows, from protein interactions and degrader development to biologics and cell therapy.
Considerations for Bioassay Design

We offer biologics development services across diverse areas such as monoclonal antibodies, T cell therapy, gene therapy, vaccines and animal health. Our services involve tailoring approaches to each modality’s unique mode of action, cell background and desired assay performance.
Assay and service considerations include optimizing cell lines, refining assay conditions, and ensuring high specificity and sensitivity to achieve accurate and reproducible results.
Bioassay Development
New Assay Design
Developing a bioassay to measure the activity of biotherapeutic candidates is a complex process that can be challenging for many laboratories. It is essential to design an assay that reflects the true mechanism of action of the drug candidate. Further, the assay must be qualified and evaluated over time to ensure maximum precision, accuracy, linearity and robustness. Our team of experts has deep experience developing potency bioassays for a wide variety of drug mechanisms of action.

Modification of Existing Bioassays
Your research may require modification of an existing bioassay. For example, needing to express a target receptor exogenously or knock out a receptor to adapt an existing bioassay for your needs. Using a variety of technologies, we can modify our comprehensive line of existing bioassays to meet your needs.
Learn more about custom bioassay design:
Antibody Characterization
Our antibody characterization services use customer-provided biologics (such as monoclonal antibodies or CAR-T cells) to generate data with our existing bioassays. These services use our off-the-shelf portfolio of:
- Immunotherapy bioassays
- PBMC ADCC bioassays
- HiBiT® Target Cell Killing assays
- Lumit® immunoassays
- PsVLP bioassays
...and many others.
Learn more about antibody characterization services:
Custom Cell Line Development and Manufacture
Consistent and reliable cell manufacturing is essential for developing and validating cell-based potency bioassays for biologic drugs. In particular, the creation and characterization of cell banks are critical processes to ensure the quality of the final product. We offer:
- Custom, bulk manufacture of cryopreserved and quality-controlled thaw-and-use cells to simplify your workflows and enable maximum assay reproducibility.
- PBMC ADCC bioassays
- CRISPR knock-ins and knock-outs
- Target cell killing (TCK) cell line engineering
- Ectopic target expression in K562 or CHO-K1 HiBiT platform
Learn more about custom cell line services:
Considerations for Target Engagement (TE) Assay Development
Our NanoBRET® Target Engagement (TE) technology offers a powerful solution for quantifying the direct binding of small molecules to target proteins within live cells. With extensive expertise in developing hundreds of assays using this technology, we recognize that we can't have an assay for every target. Work with our custom team to develop a NanoBRET® TE assay tailored to your specific needs, giving you real-time, quantitative insights into cellular target engagement to drive your drug discovery project forward.

Learn more about NanoBRET® TE assay development services and catalog products:
Custom NanoBRET® TE Assay Development Workflow
Overview

Don’t see the assay you need in our collection? Our TRS team can help! We work with you to understand the goals of your research and target proteins of interest and develop an optimized assay to meet your needs.
- Using our chemistry expertise, we build a custom NanoBRET® Tracer from your parental compound, utilizing three different linkers to ensure optimal performance. Assays built with a customer’s proprietary parental compounds are exclusively for the customer and will not be offered to others.
- We design and synthesize a custom vector, inserting your target gene(s) into a NanoLuc® fusion expression vector. Alternatively, we utilize a NanoBiT-based approach to enable the study of compound binding to protein complexes.
- We determine optimized NanoBRET® assay conditions by evaluating tracer affinity, permeability and assay window. Assays are developed to be quantitative for intracellular compound affinity (or apparent Ki) by determining the NanoBRET® Tracer concentration, which is less than or equal to Tracer Kd.
- We can optionally test known compounds to demonstrate the expected pharmacology, ensuring your assays are reliable and reproducible.
What You Receive
- A milestone report describing optimized assay conditions
- The remaining volume of each tracer
- Each NanoLuc® protein fusion vector (20µg)
Our assay protocols typically involve transient transfections in HEK293 cells, but we can also create and provide thaw-and-use (T&U) cells from the transiently transfected cell pool. T&U cells allow you to treat cells as reagents, eliminating the need to grow and transfect cells and reducing variability. If you prefer a stable cell line, we can create that for you as well.
Don’t have a parental compound? We can screen your custom vector(s) against our existing panel of tracers to identify potential binding interactions.
Learn more about custom NanoBRET® TE assay development:
Considerations for Protein:Protein Interaction (PPI) Assay Development
We offer advanced assays for measuring protein:protein interactions (PPI) in either live-cell or biochemical formats. Leveraging our innovative NanoBRET® and NanoBiT® technologies, we provide robust solutions for measuring live-cell protein interaction dynamics. Using our Lumit® technology, we can develop biochemical assays using purified proteins with common tags. Work with our team to generate customized PPI assays in your preferred format to accelerate your research.
Learn more about PPI assay development services:
Streamline Your PPI Assay Development
Overview
Protein:protein interactions (PPIs) are pivotal in numerous biological processes, ranging from transient engagements in signal transduction to the formation of complex multiprotein assemblies. To support your research, our TRS team provides specialized custom services for novel PPI assay development, using NanoBRET®, NanoBiT® and Lumit® PPI technologies.
NanoBRET® and NanoBiT® PPI assays are both cell-based solutions that allow for the measurement of protein interaction dynamics in a live cell environment. Lumit® technology provides a biochemical method for measuring a PPI between purified proteins that are fused to common protein tags.

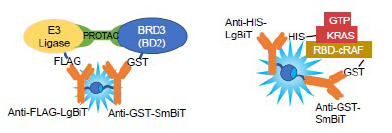
NanoBiT® PPI assays are based on the facilitated complementation of the LgBiT and SmBiT peptides. Proteins A and B are fused to LgBiT and SmBiT and expressed in cells. Interaction of fusion partners leads to structural complementation of LgBiT with SmBiT, generating a functional enzyme with a bright, luminescent signal.

NanoBRET® PPI assays are based on the energy transfer from a NanoLuc® fusion (energy donor) to a fluorescently labeled HaloTag®-Protein B fusion (energy acceptor) upon interaction of Protein A and Protein B.

In the Lumit® Anti-Tag Assays, streptavidin and antibodies against 6His, GST, DYKDDDDK (FLAG® tag) and human-Fc are labeled with LgBiT or SmBiT. When two proteins with different tags bind, the interaction can be detected using the corresponding SmBiT- and LgBiT-conjugated reagents.
Design and Development of NanoBiT® or NanoBRET® Live-Cell PPI Assays
Our custom PPI assay development process includes the following steps to build your PPI assay with the best performance characteristics.
1. Vector Synthesis: DNA vectors are designed to create fusions of the target proteins of interest with all possible tag orientations. For cytosolic proteins, this involves creating 8 fusion constructs.
- NanoBiT® PPI Assays: 4 SmBiT + 4 LgBiT protein fusions.
- NanoBRET® PPI Assays: 4 HaloTag® + 4 NanoLuc® protein fusions
Mutant controls can also be included when available. Fusions are created in bidirectional-ready vector backbones to allow for combination into a single construct.
2. Assay Development: We optimize the assay conditions by testing different tag orientations and transfection levels to obtain the combination that provides the most optimal assay window. For NanoBRET assays the optimal ratio of donor:acceptor is also determined.
3. Optional Milestones
- Compound Testing: We can test your compounds against the optimized assay to generate dose-response curves.
- Thaw-and-Use Cells: Our assay protocols typically involve transient transfections in HEK293 cells, but we can also create and provide thaw-and-use (T&U) cells from the transiently transfected cell pool.
- Stable Cell Line Development: If you prefer, we can create stable cell lines.
What You Receive
- A milestone report describing optimized assay conditions
- Each protein fusion vector (100µg)
Learn more about custom live-cell PPI assays:
Considerations for Targeted Protein Degradation (TPD) Assay Development
Streamline Your Small Molecule Degrader Development with our Customized Assay Development Services
Developing effective small molecule degraders involves understanding several key questions. At Promega, we have experience generating demonstrated assay solutions that allow for cell-based characterization of degrader molecules. Our custom assay development team collaborates closely with you to understand your research stage and specific inquiries. We design and optimize tailored assays for your unique project needs, allowing you to concentrate on generating the essential data to advance your degrader development.

Is My Target Being Degraded?
Measuring Target Protein Levels

Reporter Knock-in Cell Lines
The combination of CRISPR/Cas9 gene editing and bioluminescent protein tagging enables the development of assays for quantitative analysis of protein degrader function on endogenously expressed proteins.
- Use HiBiT knock-ins to perform quantitative end-point target protein analysis, or design the cell line to be compatible with live cell kinetics to generate key degrader metrics such as degradation rate, Dmax, DC50 and protein recovery.
- Use HaloTag®-HiBiT tandem knock-in to understand the suitability and phenotype of target protein degradation using the HaloPROTAC3 degrader.
- Use HiBiT or NanoLuc® knock-in cell lines as a starting point to further characterize ternary complex formation and target ubiquitination levels.
How We Can Help
With our technology expertise and experience in building over 250 knock-in cell lines in 50+ cell backgrounds, our custom team can build you the cell line needed to enable your degrader discovery and characterization.
Lumit® Total Protein Immunoassays
The Lumit® Immunoassay Cellular System is a no-wash bioluminescent immunoassay that can be configured to detect total protein levels in any cell background, allowing you to monitor degrader efficacy across all disease-relevant cell models.

How We Can Help
Our assay development process is milestone based. In Milestone 1 (Development) we focus on evaluating up to 16 combinations of rabbit and mouse anti-POI antibodies, together with NanoBiT®-labeled secondary antibodies, at a single concentration to identify the best-performing combination.
In Milestone 2 (Optimization), the identified antibody combination from Milestone 1 is further screened to identify the optimal primary antibody concentration. We also determine the optimal cell number as well as the linear range in the presence of a control degrader (if available).
Our assay development process is collaborative and designed to create the optimized assay to meet your experimental needs.
Learn more about Lumit® Immunoassays:
Is My Compound Cell Permeable? What Is the Binding Affinity?
Measuring Compound Affinity and Intracellular Availability

Targeted protein degraders, such as PROTACs, require binding to both the target protein of interest and the intended E3 ligase to drive degradation. In addition, compound effectiveness is dependent on compound permeability, which can be challenging for the hetero-bifunctional PROTAC compounds that are large and “beyond rule of 5”, when compared to classical small-molecule inhibitors.
NanoBRET® TE Technology provides real-time insights into how targeted protein degraders interact with their target proteins or intended E3 ligase in live cells. Using a bioluminescence resonance energy transfer (BRET) technique, these assays measure the binding affinity of degraders to either the target protein or E3. In addition, the assays enable the assessment of the apparent cellular affinity in live cells and the intrinsic binding affinity in permeabilized cells, where the plasma membrane barrier is removed. This dual-mode approach allows for a comprehensive evaluation of a degrader’s intracellular availability—an essential factor for optimizing the design and efficacy of these compounds.
How We Can Help
Work with our custom assay team to design a NanoBRET TE assay for either your target protein or E3 ligase.
Learn more about assays for cell permeability and binding affinity:
Does My Compound Force the Required Protein Interactions?
Measuring Ternary Complex Formation and Target Ubiquitination
Inducing a protein-protein interaction is fundamental to the function of PROTACs and other degrader compounds. They facilitate the recruitment of an E3 ubiquitin ligase to the target protein to generate a ternary complex, resulting in target protein ubiquitination and subsequent degradation by the proteasome when the ternary complex is productive. NanoBRET® PPI Technology allows for the measurement of ternary complex and ubiquitination within a live-cell environment, allowing researchers to:
- Optimize the design of degraders to enhance their potency, selectivity, and overall efficacy in degrading the intended target proteins
- Fine-tune the molecular structure of degraders to improve their therapeutic potential
- Confirm proposed mechanisms of action
How We Can Help
Work with our custom assay development team to develop assays for either ternary complex or target protein ubiquitination using NanoBRET® PPI technology.
- Develop the assay starting with endogenous HiBiT-tagged proteins. We start with a CRISPR engineered cell line with HiBiT fused to the target protein of interest. Transient transfection conditions are determined for the target E3 ligase/ubiquitin.
- Develop the assay in transient format. We work in HEK293 cells and develop the optimal vectors and transfection conditions for your target protein and E3 ligase/ubiquitin. We primarily develop assays based on VHL and CRBN E3 ligases but can also work with novel E3s upon request.

What Are the Cellular Consequences of Target Degradation?
Understanding Degradation Phenotype

Often the first step in degrader compound development is understanding how loss of the target protein will impact the cellular response. As an alternative to protein knock-out, HaloPROTAC3 is a small molecule degrader that can be used to drive targeted degradation of proteins expressed as HaloTag® fusions to investigate the cellular phenotype of protein loss. When proteins are tandem tagged with HaloTag-HiBiT, target protein levels can be easily quantified following HaloPROTAC3 treatment, allowing for correlation between target protein levels and observed cellular response.
How We Can Help
With our expertise in HaloTag® and HiBiT technologies and experience in building over 250 knock-in cell lines, our custom team can build you the HaloTag-HiBiT knock-in cell line needed to enable the characterization of target protein loss and help you further understand the biology of the protein being studied.
Learn more about HaloTag®-HiBiT cell lines to study degradation phenotype:
Considerations for Lumit® Immunoassay Development
Lumit® Immunoassays provide a simple and fast alternative to conventional immunoassay methods, including sandwich ELISAs and Western blots. The bioluminescence-based method can be completed in as little as 30 minutes. We can develop custom Lumit® immunoassays to to measure the abundance of analytes, post-translational protein modifications, and protein:protein interactions.
Lumit® Assay Development Workflow
Assay Development
- Client provides a description of the desired assay and intended use.
- TRS will schedule a call to get more details, if needed.
- TRS will propose assay options with ballpark pricing and timeline to Client.
- TRS will write a statement of work (SOW) for the preferred option.
Antibody/Analyte Labeling
- Client provides material in a suitable format for labeling.
- TRS performs labelling and confirms via gel analysis.
- Client receives all labeled materials.
Lumit® Assay Development: Formats

Direct format uses labeled primary antibodies. Catalog examples include the Lumit® Cytokine Assays.

Indirect format uses labeled secondary antibodies. Catalog examples include the Lumit® Immunoassay Cellular Systems.

Competitive format uses a labeled analyte and tracer. Catalog examples include the Lumit® FcRn Assay.
Lumit® Assay Development: Other Examples

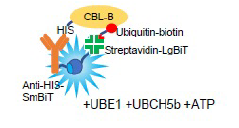
Auto-ubiquitination assays using anti-tag antibodies, biotinylated ubiquitin and streptavidin
Lumit® Assay Development: Antibody Labeling
