What are Seroprevalence Surveys and What Do They Tell Us About COVID-19?
By: Emily A. Teslow, Ph.D., Medical Affairs • October 2020
COVID-19 Prevalence and the Key Role of Antibody Testing
The outbreak of COVID-19, caused by the novel infectious disease agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or 2019-nCoV, began around December 2019 in Wuhan Hubei Province China1,2. As of September 2020, there were over 30 million confirmed COVID-19 cases reported worldwide, a number which will likely continue to increase in certain populations3. Since the start of this pandemic scientists have learned that the range of disease manifestations and immune responses, which occur after infection with SARS-CoV-2, vary significantly. We now know that many individuals present with either asymptomatic or mild disease4-11, and that the number of laboratory confirmed cases provides an inaccurate measure of true disease prevalence as many of these cases have not yet been captured by conventional diagnostic testing methods. Antibody or serologic testing has played an important role in our understanding of disease prevalence thus far and may continue to be crucial in addressing these concerns.
Serologic tests, which detect the presence of antibodies produced against a pathogen within an individual’s blood, indicate whether an individual has previously been infected by the SARS-CoV-2 virus. Serological data has been collected for the SARS-CoV-2 virus using several different types of assay technologies including enzyme-linked immunosorbent assays (ELISA)12-16, chemiluminescent immunoassays17-21, immunofluorescence assays22, lateral flow assays23, and bioluminescent immunoassays (BLIA)24-26. These types of tests, provided they have been well validated and have high clinical performance, are essential to understanding the impact of disease around the world.
Seroprevalence is the proportion of individuals within a population affected by disease at a specific time point, based on positive testing for serum antibodies.
COVID-19 Seroprevalence Surveys and Zoonotic Transmission
Globally researchers and public health organizations are exploring strategies to better understand the spread of COVID-19 disease, using seroprevalence as a critical measure. Seroprevalence is the proportion of individuals within a population affected by disease at a specific time point, based on positive testing for serum antibodies27. Due to resource and time constraints, it is challenging if not impossible to test every individual within a population. To estimate the percentage of infected individuals within a population (e.g., country, state, community, etc.) seroprevalence survey studies or “serosurveys” are being developed. A serosurvey can be performed by sampling small numbers of participants within a populace, which represent the general population. When using a serologic test with high sensitivity and specificity, serosurveys provide essential information about true disease prevalence. These types of surveys are already being performed on a large scale by the United States Centers for Disease Control and Prevention28, the Spanish Ministry of Health29, and many other government bodies30. Serosurveys also allow us to measure disease prevalence in specific communities or populations such as schools31,32, hospital workers33-35, military officials36, nursing homes37 or prisons38. For example, the UK government recently launched a serosurvey to assess and monitor the prevalence of COVID-19 among pre-school, primary and secondary school pupils and teachers32.
Evaluating the true magnitude of an outbreak is vital to establishing public health measures and control strategies. Seroprevalence surveys allow public health officials and policymakers to identify hot spots and areas where transmission has been reduced so that they may respond accordingly. Serologic data may also provide guidance to governing bodies for instituting public health strategies including vaccine procurement/prioritization, allocation of resources (PPE, financial support, etc.), and establishment of mitigation tactics.
In addition to monitoring disease spread in humans, serosurveys are used to monitor seropositivity of animal reservoirs, which are populations of organisms in which an infectious disease agent survives and replicates. These animal reservoirs are important, as they may be able to transmit virus directly to humans. For example, the 2003 outbreak of SARS-CoV was thought to have originated from the palm civet animal reservoir, as 99.6% of the viral genome isolated from civets was homologous to the SARS-CoV viral genome extracted from humans. In this case the government instituted strict policies, leading to the elimination/quarantine of all domesticated civets for human consumption, after which there were no new community acquired cases reported in China39. Studies are ongoing to determine if there are important animal reservoirs that may have facilitated zoonotic transmission of SARS-CoV-240. However recent studies have reported that pet animals, including cats and dogs, have been testing positive for SARS-CoV-2 antibodies41. Only a few of the human serological assays developed so far can be used in animals. For example, assays utilizing NanoBiT® technology, in which viral antigens coupled with bioluminescent enzyme fragments are used to detect anti-SARS-CoV-2 antibodies, can be performed irrespective of species origin25,26.
As government officials, healthcare organizations, and other institutions face an unprecedented global health crisis, determining seroprevalence for COVID-19 in both humans and animals will be an essential and necessary tool in facing the multitude of challenges ahead.
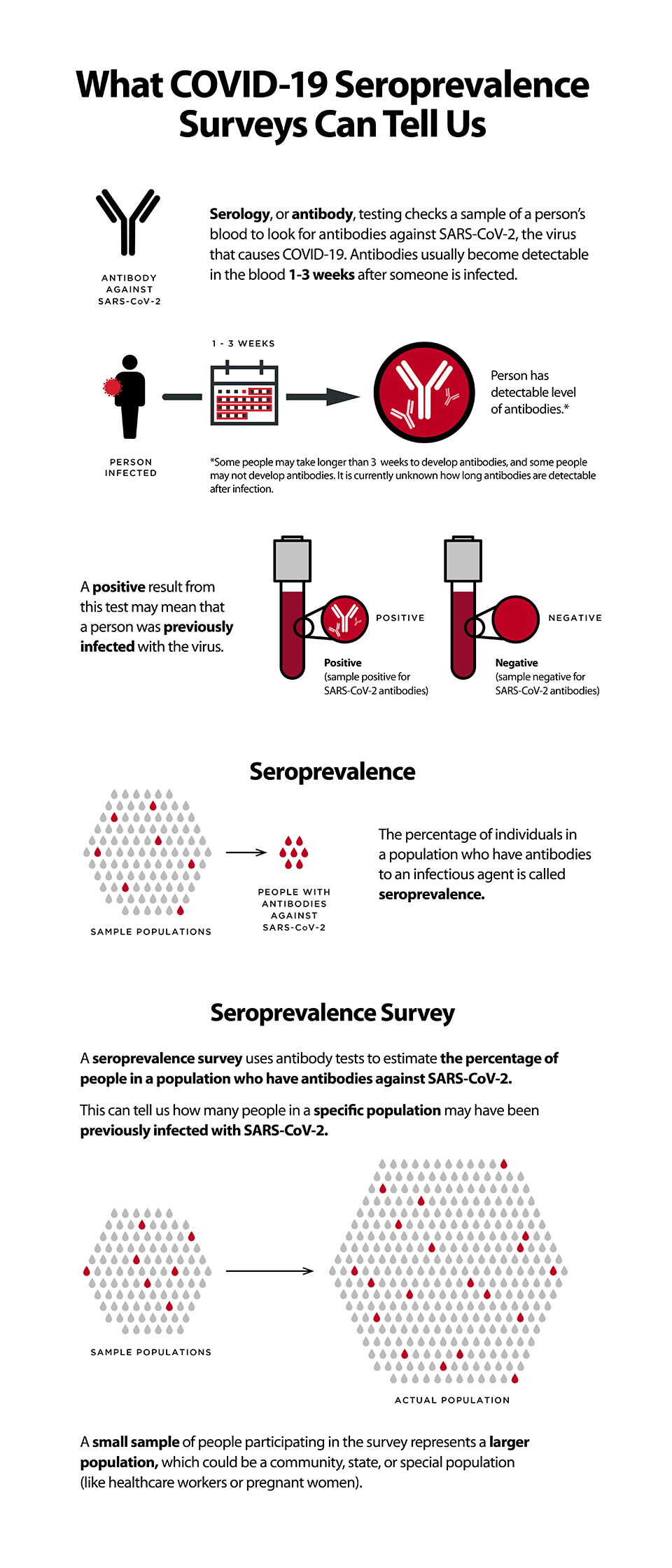
Image Source: CDC What COVID-19 seroprevalence surveys can tell us.
Reference to specific commercial products, manufacturers, companies, or trademarks does not constitute its endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention.
References:
- She, J. et al. (2020) 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin. Transl. Med. 9, 19, doi:10.1186/s40169-020-00271-z .
- Chen, Y., Liu, Q. and Guo, D. (2020) Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 92, 418-423, doi:10.1002/jmv.25681.
- JHU. (2020) COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University & Medicine, https://coronavirus.jhu.edu/map.html
- Kimball, A. et al. (2020) Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 377-381, doi:10.15585/mmwr.mm6913e1.
- Lai, C. C. et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J. Microbiol. Immunol. Infect. doi:10.1016/j.jmii.2020.02.012 (2020).
- Lohse, S. et al. (2020) Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect. Dis., doi:10.1016/S1473-3099(20)30362-5.
- Mizumoto, K., Kagaya, K., Zarebski, A. & Chowell, G. (2020) Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro. Surveill. 25, pii=2000180, doi:10.2807/1560-7917.
- Wu, X., et al. (2020) Serological tests facilitate identification of asymptomatic SARS-CoV-2 infection in Wuhan, China. J. Med. Virol., doi:10.1002/jmv.25904.
- Choe, P. G. et al. (2020) Antibody Responses to SARS-CoV-2 at 8 Weeks Postinfection in Asymptomatic Patients. Emerging inf. Dis. 26, doi:10.3201/eid2610.202211.
- Liu, X. et al. (2020) Serum IgM against SARS-CoV-2 correlates with in-hospital mortality in severe/critical patients with COVID-19 in Wuhan, China. Aging (Albany NY) 12, 12432-12440, doi:10.18632/aging.103417.
- Guan, W.-j. et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, doi:10.1056/NEJMoa2002032.
- Amanat, F. et al. (2020) A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med., doi:10.1038/s41591-020-0913-5.
- Perera, R. A. et al. (2020) Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro. Surveill. 25, doi:10.2807/1560-7917.Es.2020.25.16.2000421.
- Stadlbauer, D. et al. (2020) SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr .Protocols Microbiol. 57, e100, doi:10.1002/cpmc.100.
- Zhao, J. et al. (2020) Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clinical Infectious Diseases, doi:10.1093/cid/ciaa344.
- Liu, L. et al. (2020) A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes and Infection, doi:10.1016/j.micinf.2020.05.008.
- Cai, X. F. et al. (2020) A Peptide-based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Coronavirus Disease 2019 (COVID-19). J. Infect. Dis., doi:10.1093/infdis/jiaa243.
- Qu, J. et al. (2020) Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis., doi:10.1093/cid/ciaa489.
- Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465-469, doi:10.1038/s41586-020-2196-x (2020).
- Tré-Hardy, M. et al. (2020) Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin. Chem. Lab. Med., doi:10.1515/cclm-2020-0594.
- Ma, H. et al. (2020) Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol. Immunol., doi:10.1038/s41423-020-0474-z.
- Long, Q.-X. et al. (2020) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine, doi:10.1038/s41591-020-0897-1.
- Li, Z. et al. (2020) Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol., doi:10.1002/jmv.25727.
- Burbelo, P. D. et al. (2020) Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients. J. Infect. Dis., doi:10.1093/infdis/jiaa273.
- Elledge, S. K. et al. (2020) Engineering luminescent biosensors for point-of-care SARS-CoV-2 antibody detection. medRxiv, doi:10.1101/2020.08.17.20176925.
- Kainulainen, M. H. et al. (2020) High-throughput quantitation of SARS-CoV-2 antibodies in a single-dilution homogeneous assay. medRxiv, 2020.2009.2016.20195446, doi:10.1101/2020.09.16.20195446.
- Sood, N. et al. (2020) Seroprevalence of SARS-CoV-2-Specific Antibodies Among Adults in Los Angeles County, California, on April 10-11. JAMA, doi:10.1001/jama.2020.8279.
- CDC. (2020) Large-scale Geographic Seroprevalence Surveys.
- Pollán, M. et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England) 396, 535-544, doi:10.1016/s0140-6736(20)31483-5 (2020).
- Eckerle, I. and Meyer, B. (2020) SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet (London, England) 396, 514-515, doi:10.1016/s0140-6736(20)31482-3 (2020).
- Torres, J. P. et al. SARS-CoV-2 antibody prevalence in blood in a large school community subject to a Covid-19 outbreak: a cross-sectional study. Clin. Infect. Dis., doi:10.1093/cid/ciaa955.
- UK.GOV. (ed Department of Health and Social Care) (UK Government 2020).
- Quattrone, F. et al. (2020) The value of hospital personnel serological screening in an integrated COVID-19 infection prevention and control strategy. Infect. Control Hosp. Epidemiol., 1-2, doi:10.1017/ice.2020.242.
- Garcia-Basteiro, A. L. et al. (2020) Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 11, 3500, doi:10.1038/s41467-020-17318-x.
- Self, W. H. et al. (2020) Seroprevalence of SARS-CoV-2 Among Frontline Health Care Personnel in a Multistate Hospital Network - 13 Academic Medical Centers, April-June 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1221-1226, doi:10.15585/mmwr.mm6935e2.
- Payne, D. C. et al. (2020) SARS-CoV-2 Infections and Serologic Responses from a Sample of U.S. Navy Service Members - USS Theodore Roosevelt, April 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 714-721, doi:10.15585/mmwr.mm6923e4.
- Ladhani, S. N. et al. (2020) High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19; a prospective cohort study in England. medRxiv, 2020.2008.2010.20171413, doi:10.1101/2020.08.10.20171413.
- Saloner, B., et al. (2020) COVID-19 Cases and Deaths in Federal and State Prisons. JAMA 324, 602-603, doi:10.1001/jama.2020.12528.
- Shi, Z. & Hu, Z. (2008) A review of studies on animal reservoirs of the SARS coronavirus. Virus Research 133, 74-87, doi:10.1016/j.virusres.2007.03.012.
- Gautam, A., et al. (2020) Susceptibility to SARS, MERS, and COVID-19 from animal health perspective. Open Vet J 10, 164-177, doi:10.4314/ovj.v10i2.6.
- Hossain, M. G., et al. (2020) SARS-CoV-2 host diversity: An update of natural infections and experimental evidence. J. Microbiol. Immunol. Infect. doi: 10.1016/j.jmii.2020.06.006
Coronavirus Products and Support
Information on testing for COVID-19 infection and antibodies is available on our SARS-CoV-2 Serology and PCR Testing page.