Diamond™ Nucleic Acid Dye: A Sensitive Alternative to SYBR® Dyes
Alisha Truman, Brad Hook and Amy Hendricksen
Promega Corporation
Abstract
In this study, we evaluated the Diamond™ Nucleic Acid Dye, SYBR® Gold Nucleic Acid Gel Stain and SYBR® Safe DNA Gel Stain for detecting both DNA and RNA in agarose gels. The dyes were used as either a gel stain, a precasting agent or were preloaded directly into sample loading buffer. We found that using the Diamond™ Nucleic Acid Dye as a gel stain offered the best results over a wide range of nucleic acid concentrations and molecular weight fragments.
Introduction
Visualization of nucleic acids via gel electrophoresis is a very common method for evaluating nucleic acid quality, quantity and fragment size. This process has been routinely used since the late 1960s, and ethidium bromide is a commonly used chemical for staining nucleic acids within the gel matrix. Ethidium bromide is an intercalating agent and, given its mechanism of action, is a mutagenic substance.
Several alternatives to ethidium bromide have been developed. These alternatives are not intercalating agents; rather, they are fluorescent agents that bind to the grooves of DNA. Aside from the improved safety, some of these agents show improved sensitivity when used to stain nucleic acids in gels. The nucleic acid gel staining dye, Diamond™ Nucleic Acid Dye (Cat.#H1181), also can be used to visualize DNA and RNA in gels. This dye can be used on agarose and polyacrylamide gels, is compatible with many commonly used buffers and can be used in a precast gel system. The Diamond™ Nucleic Acid Dye was developed to provide researchers with a safe and sensitive replacement for ethidium bromide. Like the highly sensitive SYBR® Gold and SYBR® Safe stains, Diamond™ Nucleic Acid Dye has the potential to alter the migration pattern of nucleic acids within a gel matrix if used as a preloading agent or it may alter electrophoretic mobility of the nucleic acids when used with a precast gel. SYBR® Gold and SYBR® Safe stains makes claims that these dyes can be used as gel stains, precasting dyes or added directly to samples when loading the gel. We have evaluated the two SYBR® stains as well as Diamond™ Nucleic Acid Dye for their performance in detecting both DNA and RNA in agarose gels when used as either a gel stain, precasting agent or preloaded directly into sample loading buffer.
Materials and Methods
Nucleic Acid Dye
- Diamond™ Nucleic Acid Dye (Cat.# H1181)
- SYBR® Gold Nucleic Acid Gel Stain (10,000X concentrate in DMSO; Life Technologies, Cat.# S-11494)
- SYBR® Safe DNA Gel Stain (Life Technologies, Cat.# S-33102)
Markers
- BenchTop 1kb DNA Ladder (Cat.# G7541)
- RNA Markers (Cat.# G3191)
Buffers
- TAE Buffer, 10X Molecular Biology Grade (Cat.# V4271)
- TE Buffer, 1X Molecular Biology Grade (Cat.# V6232)
Agarose
- Agarose, Low Melting Point, Analytical Grade (Cat.# V2111)
Imaging Equipment
- Molecular Imager® Gel Doc™ XR System (Bio-Rad, Cat.# 170-7950EDU )
Dilution of Nucleic Acids
DNA: Four-fold serial dilutions of BenchTop 1kb DNA Ladder were prepared in 1X Blue/Orange Loading Dye
(Cat. #G1881)
RNA: Two-fold serial dilutions of RNA Markers were prepared in TE Buffer.
Gel Staining Method
For all DNA sample, 10µl (100ng/µl) was run per lane in 0.8% agarose gels. Gels containing DNA samples were run for 45 minutes at 100 volts. For all RNA samples, 3µl of RNA was added to 3µl of formaldehyde loading dye. RNA samples were incubated for 5 minutes at 70°C and then run on a 1% agarose gel for 60 minutes at 75 volts.
All stains are provided as 10,000X stock concentrations in DMSO. After electrophoresis, all gels were stained in a 1:10,000 dilution of due for 30 minutes, protected from light.
Precasting Method
DNA and RNA samples were prepared as previously described. Concentrated stains were diluted 1:10,000 in molten agarose (0.8% for DNA and 1% for RNA) prior to casting. Gels were run, protected from light, for 45 minutes at 100 volts for DNA samples and 60 minutes at 100 volts for RNA samples.
Preloading Method (DNA Only)
DNA samples were prepared as previously described. Dyes were diluted in 1X Blue/Orange Loading Dye to a final dilution of 1:10,000 for DNA samples. Gels were run protected from light for 45 minutes at 100 volts.
Visualization of Nucleic Acids
All gels were imaged on the Molecular Imager® Gel Doc™ XR System using the SYBR® Gold or the SYBR® Safe setting.
Results
DNA Detection
As a gel stain for DNA, Diamond™ Nucleic Acid Dye performed similarly to SYBR® Gold Nucleic Acid Gel Stain and was more sensitive than SYBR® Safe DNA Gel Stain (Figure 1, Panel A). In precast gels, Diamond™ Nucleic Acid Dye and SYBR® Gold Nucleic Acid Gel Stain stained all DNA bands, but due to exposure effects, only concentrations in the middle dilution range were effectively visualized (Figure 1, Panel B). High concentrations of samples stained with the Diamond™ Nucleic Acid Dye and SYBR® Gold Nucleic Acid Gel Stain yielded bright smears that made individual bands indistinguishable. In contrast, the lowest dilutions were not visible. This indicates that it is necessary to optimize nucleic acid concentrations and load volumes when using Diamond™ Nucleic Acid Dye in precast gels.
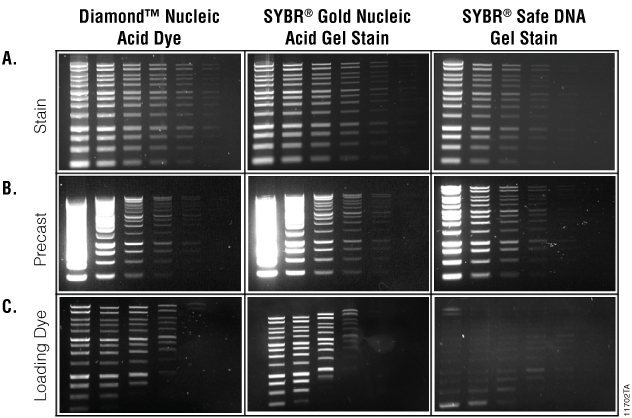
Preloading samples with the nucleic acid dyes also yielded results similar to those in precast gels. Samples preloaded with the Diamond™ Nucleic Acid Dye showed a loss of sensitivity with low concentrations of small molecular weight DNA, and DNA migration was affected but less so than samples preloaded with SYBR® Gold Nucleic Acid Gel Stain. Samples preloaded with SYBR® Safe DNA Gel Stain yielded poor results at all concentrations (Figure 1, Panel C).
RNA Detection
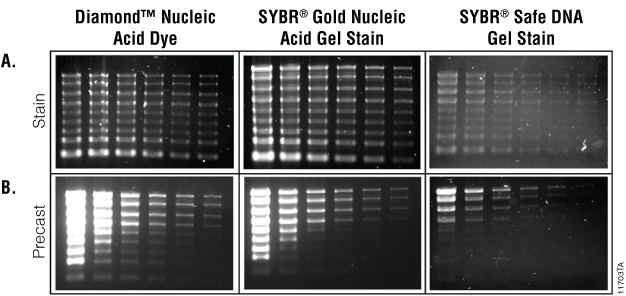
When used as a gel stain with RNA samples, the Diamond™ Nucleic Acid Dye and the two SYBR® dyes tested showed comparable results, staining all RNA bands (Figure 2, Panel A). Precasting gels with these dyes resulted in only RNA concentrations in the middle sample input range being effectively visualized (Figure 2, Panel B). Higher concentrations of sample stained with the Diamond™ Nucleic Acid Dye and SYBR® Gold Nucleic Acid Gel Stain produced a bright smear that made individual bands indistinguishable, and the lower concentrations and lower molecular weight fragments were not visible at the gel imaging setting used. Adding dye to the RNA sample loading buffer resulted in no detection of visible bands regardless of the dye tested (data not shown). We believe this is due to the 70°C incubation in the presence of formaldehyde.
Conclusion
We found that the Diamond™ Nucleic Acid Dye performed comparably to SYBR® Gold and SYBR® Safe as a gel stain for both DNA and RNA. For visualization of DNA across a range of concentrations, the Diamond™ Nucleic Acid Dye can be used at a 1:10,000 dilution when precasting gels but may require optimization. Diamond™ Nucleic Acid Dye also can be used as an addition to the loading buffer; however, detection of low concentrations of small molecular weight DNA is diminished and DNA migration is affected. This also was observed with SYBR® Gold Nucleic Acid Gel Stain. Diamond™ Nucleic Acid Dye consistently yielded better results than SYBR® Safe DNA Gel Stain under all experimental conditions tested. Overall, the Diamond™ Nucleic Acid Dye offered sensitivity for detecting both DNA and RNA over a wide range of concentrations and across a range of molecular weights. For best results, we recommend using Diamond™ Nucleic Acid Dye as a gel stain so that mobility of nucleic acid fragments and detection of faint or low molecular weight bands is not affected. Using the dye in this fashion minimizes the need for nucleic acid concentration optimization, reduces costs and saves time.