Human Macrophages, ADCP-Qualified
| Catalog No. | Product Name | Size | Price | Qty | |||
|---|---|---|---|---|---|---|---|
| CS3055C02 | Early Access Human Macrophages, ADCP-Qualified, 1X | View Specifications | 1 each | Please Enquire | |||
| CS3055C03 | Early Access Human Macrophages, ADCP-Qualified, 5X | View Specifications | 1 each | Please Enquire |
Early Access = This product is available under our Early Access program - Learn More
Catalog (FT) = This product is available under our Catalog (FT) program - Learn More
Human T Cell (CD8+), TDCC-Qualified
| Catalog No. | Product Name | Size | Price | Qty | |||
|---|---|---|---|---|---|---|---|
| CS3055B03 | Early Access Human T Cell (CD8+), TDCC-Qualified | View Specifications | 1 each | Please Enquire | |||
| CS3055B06 | Early Access Human T Cell (CD8+) TDCC-Qualified 5X | View Specifications | 1 each | Please Enquire |
Early Access = This product is available under our Early Access program - Learn More
Catalog (FT) = This product is available under our Catalog (FT) program - Learn More
Human PBMC, ADCC-Qualified
| Catalog No. | Product Name | Size | Price | Qty | |||
|---|---|---|---|---|---|---|---|
| CS3055A15 | Early Access Human PBMC, ADCC-Qualified | View Specifications | 1.0ml | Please Enquire | |||
| CS3055A18 | Early Access Human PBMC, ADCC-Qualified 5X | View Specifications | 1 each | Please Enquire |
Early Access = This product is available under our Early Access program - Learn More
Catalog (FT) = This product is available under our Catalog (FT) program - Learn More
Qualified Primary Cells: Minimize Variability, Enhance Reliability
Promega primary effector cells provide a consistent and robust method for target cell killing. Our primary cells are biologically relevant and MoA-qualified for either ADCC, ADCP or TDCC activity to measure the potency and stability of antibodies and other biologics that specifically bind and activate their respective effector cells. These primary effector cells are functionally tested with our HiBiT Target Cell Killing Bioassays, where upon lysis of the target cells and addition of detection reagent, specific cell killing of the target cell is measured by a bright, bioluminescent signal.
Human PBMC, ADCC-Qualified:
Eliminate the Uncertainty and Variability of Primary Cell-Based ADCC Assays
Our thaw-and-use ADCC-Qualified PBMCs provide consistency and robustness for measuring antibody-dependent cellular cytotoxicity. The PBMCs are available in two product formats: as standalone vials or bioassay kits.
Standalone Human PBMC, ADCC-Qualified Cells
- Single-donor-derived PBMC lots
- FcγR-genotyped for FcγRIIIa and FcγRIIa
- ADCC-qualified with HiBiT target cell lines
- Thaw-and-use: No culture required

Representative cellular profiles of ADCC-qualified PBMC donors. Our lots of PBMCs reflect the donor-to-donor variability expected in these sample types. This variability is crucial for understanding real-world immune responses when developing therapeutics.
PBMC ADCC Bioassay: PBMCs Paired with a Selection of HiBiT Target Cell Killing Bioassays
The PBMC ADCC Bioassay provides ADCC-qualified PBMC effector cells and a choice of popular target cells expressing a HiBiT fusion protein. The bioassay is simple, homogenous, highly sensitive and provides a robust assay window.

In addition to FcyR genotyping, our bioassay kits are functionally tested using our HiBiT Target Cells to demonstrate ADCC activity. Representative bioassay data using our Raji (HiBiT) Target Cells are shown.
Click on your target cell of interest to find the PBMC ADCC Bioassay for you:
Each PBMC Bioassay kit includes Human PBMCs, ADCC-Qualified; selected Target Cells; RPMI 1640 Media; Fetal Bovine Serum; and Bio-Glo-NB™ TCK Luciferase Assay System.
Human T Cell (CD8+), TDCC-Qualified: Improve Your T Cell Cytotoxicity Assays
Our TDCC-qualified CD8+ T cells provide consistency and robustness for measuring T cell-dependent cellular cytotoxicity. The T cells are available in two product formats: as standalone vials or in a bioassay kit.
Standalone Human T Cell (CD8+), TDCC-Qualified Cells
- Single-donor-derived T cells
- TDCC-qualified with HiBiT target cell lines
- Thaw-and-use: No culture required
Each Donor is Characterized for CD8+ T Cell Purity Following Expansion
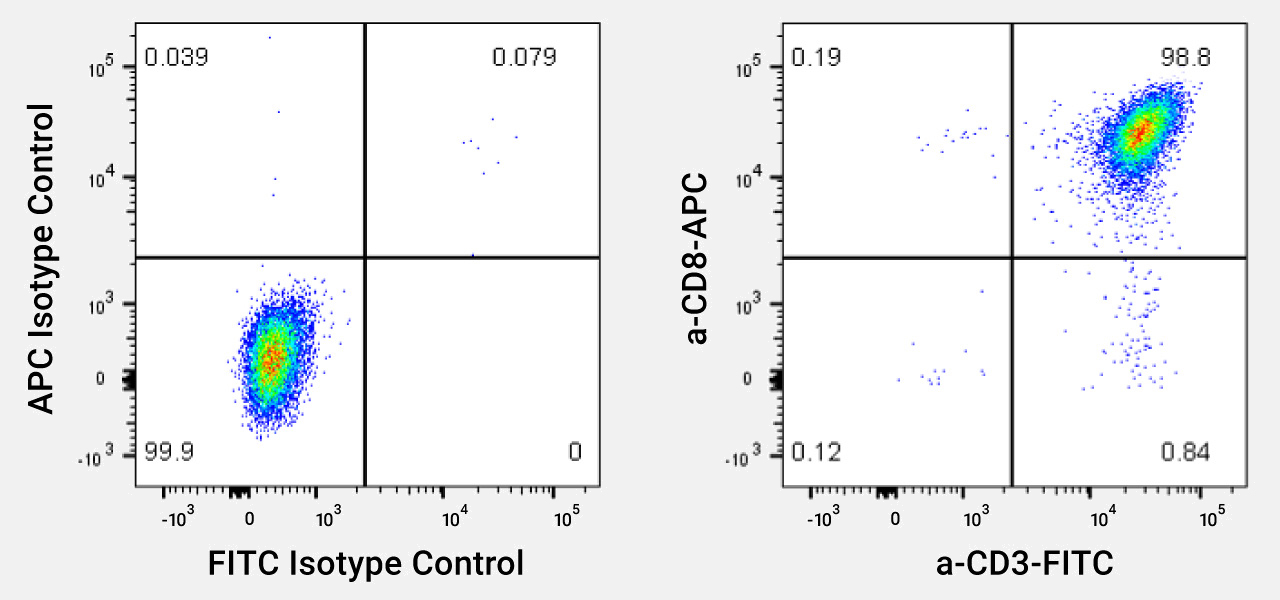
Representative profile of CD8 T Cells (TDCC-Qualified). Cells were thawed and stained with antibodies against CD3 and CD8+ (or isotype controls.) Cell populations are >98% CD8+ T Cells.
CD8+ TDCC Bioassay: CD8+ T Cells Paired with a Selection of HiBiT Target Cell Killing Bioassays
The CD8+ TDCC Bioassay, using engineered HiBiT target cells, measures the antibody potency and specific target cell killing. The bioassay is simple, homogenous, highly sensitive and provides a robust assay window.

Our bioassay kits are functionally tested using our HiBiT Target Cells to demonstrate TDCC activity. Representative assay data using our Raji (HiBiT) Target Cells are shown.
Click on your target cell of interest to find the CD8+ TDCC Bioassay for you:
Each TDCC Bioassay kit includes Human CD8+ T cells, TDCC-Qualified; selected Target Cells; RPMI 1640 Media; Fetal Bovine Serum; and Bio-Glo-NB™ TCK Luciferase Assay System.
Human Macrophages, ADCP-Qualified: Off-the-Shelf Primary Macrophage
Our ADCP-qualified primary macrophage provide consistency and robustness for measuring antibody-dependent cellular phagocytosis. The macrophage are available as standalone thaw-and-use vials.
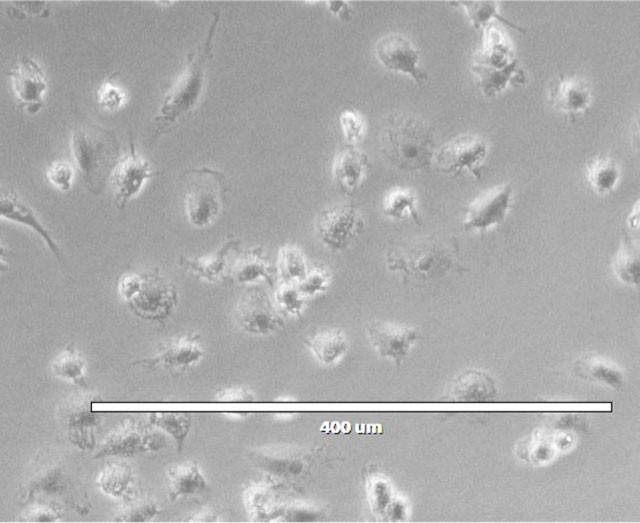
Standalone Human Macrophages, ADCP-Qualified Cells
- Derived from primary human monocytes
- Optimized for ADCP activity with HiBiT target cells
- Large batch sizes for consistent performance
- High viability (>95%) and flexible phenotype after thaw
- Loss-of-signal assay correlates with macrophage ADCP mechanism of action
| Upregulated | Downregulated | No Change |
|---|---|---|
| HLA-DR | CD68 | FcγRII |
| CD80 | FcγRIII | |
| CD86 | CD163 | |
| CCR7 | CD206 | |
| CD40 | SIRPα | |
| FcγRI |
Human Macrophages (ADCP-Qualified) were thawed and cultured overnight with 50ng/ml IFNγ. Images were collected on an EVOS M5000. The table shows cellular markers that are upregulated/downregulated/unchanged after IFNγ treatment, relative to expression immediately after thaw.
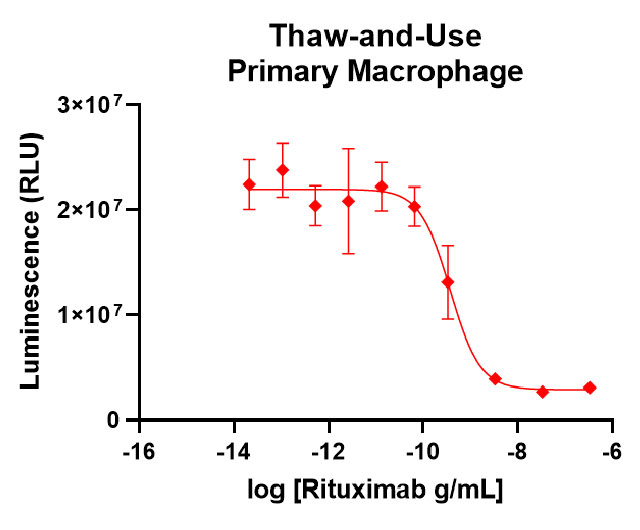
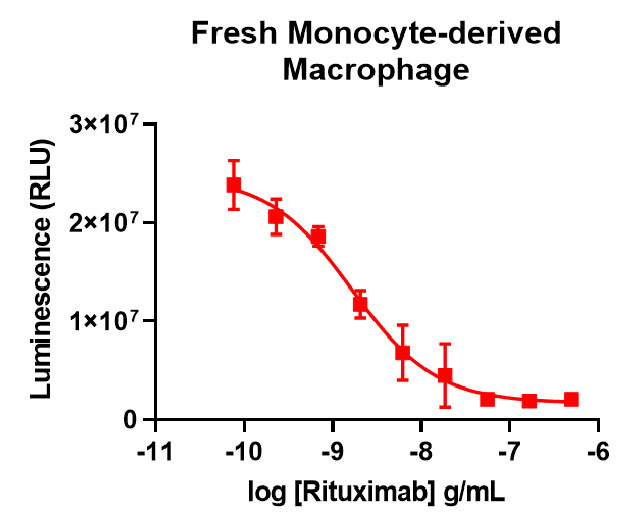
Our thaw-and-use primary macrophage (left panel) provide a quality of antibody-dependent cellular phagocytosis data that is consistent with or better than what researchers see using other sources of macrophage, such as monocyte-derived (right panel).
Protocols
No protocols available
Specifications
Catalog Number:
What's in the box?
| Item | Part # | Size |
|---|---|---|
Human Macrophages (ADCP-Qualified) |
CS3055C01 | 1 × 0.5ml |
Promega Cell Line Limited Use Label License (LULL)
BY USE OF THIS MATERIAL, RECIPIENT AGREES TO BE BOUND BY THE TERMS OF THIS LIMITED USE LABEL LICENSE.
-
1. Usage and Transfer Restrictions:
- 1.1. Recipient may use this material for research use only, which includes but is not limited to drug discovery and development, quality assurance testing and product release assays. No transfer, resale or commercial use of this material is allowed without the written consent of Promega Corporation.
- 1.2. “Commercial use” means any and all uses of this material or derivatives by recipient for monetary or other consideration, including product manufacture, providing a service, information or data to unaffiliated third parties and resale of the material for any use.
-
2. Modification Restrictions:
- 2.1. Recipient shall have no right to modify, derivatize or otherwise create variations of the nucleotide sequence of any proprietary gene, including but not limited to luciferase, NanoBiT® technology (e.g., HiBiT), HaloTag® technology or genes stably transfected within the cells.
- 2.2. Recipient shall have no right to genetically engineer or otherwise modify the cell line.
-
3. Propagation and Storage:
- 3.1. The recipient may not propagate the cells, unless material is marked as Propagation Model, Cell Bank or Master Cell Bank; then recipient may propagate and store the cells for long-term use.
-
4. For all determinations of luminescence or other assay-related activity of this material and its derivatives, recipient must either:
- 4.1. Use the detection systems specified in the protocol or data report and purchased from Promega for all luminescence assays when using this material; or
- 4.2. Contact Promega to obtain a license for use of this material with reagents other than a Bio-Glo™ branded luciferase reagent, including but not limited to:
- 4.2.1 Bio-Glo™, Bio-Glo-NL™, Bio-Glo-NB™ TCK, Bio-Glo-NB™ VLP, Bio-Glo-NB™ Lytic
-
5. For uses of this material for energy transfer (such as bioluminescence resonance energy transfer), recipient must:
- 5.1. Use NanoBRET™-branded luminescent assay reagents (LARs; e.g., NanoBRET™ Nano-Glo® Substrate), Intracellular TE Nano-Glo® Substrate/Inhibitor or Intracellular TE Nano-Glo® Vivazine™/Inhibitor for all determinations of luminescent activity by this material and its derivatives; and
- 5.2. Use NanoBRET™-branded energy acceptors (e.g., NanoBRET™ tracers, NanoBRET™ dyes, BRET-optimized HaloTag® ligands) for all determinations of energy transfer activity by this material and its derivatives; or
- 5.3. Contact Promega to obtain a license for the use of the material and its derivatives for energy transfer assays to energy acceptors not manufactured by Promega. No license is needed if the energy transfer acceptor is a genetically encoded autofluorescent protein.
-
6. For uses of HaloTag® Technology in this material, recipient must either:
- 6.1. Use Promega HaloTag® ligands, which can be modified or linked to Promega or customer-supplied moieties; or
- 6.2. Contact Promega to obtain a license if Promega HaloTag® ligands are not to be used.
-
7. Disclaimer and Governing Law:
- 7.1. PROMEGA MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING FOR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH REGARD TO THE MATERIAL. The terms of this label license shall be governed under the laws of the State of Wisconsin, USA.
What's in the box?
| Item | Part # | Size |
|---|---|---|
Human Macrophages (ADCP-Qualified) |
CS3055C01 | 5 × 0.5ml |
Promega Cell Line Limited Use Label License (LULL)
BY USE OF THIS MATERIAL, RECIPIENT AGREES TO BE BOUND BY THE TERMS OF THIS LIMITED USE LABEL LICENSE.
-
1. Usage and Transfer Restrictions:
- 1.1. Recipient may use this material for research use only, which includes but is not limited to drug discovery and development, quality assurance testing and product release assays. No transfer, resale or commercial use of this material is allowed without the written consent of Promega Corporation.
- 1.2. “Commercial use” means any and all uses of this material or derivatives by recipient for monetary or other consideration, including product manufacture, providing a service, information or data to unaffiliated third parties and resale of the material for any use.
-
2. Modification Restrictions:
- 2.1. Recipient shall have no right to modify, derivatize or otherwise create variations of the nucleotide sequence of any proprietary gene, including but not limited to luciferase, NanoBiT® technology (e.g., HiBiT), HaloTag® technology or genes stably transfected within the cells.
- 2.2. Recipient shall have no right to genetically engineer or otherwise modify the cell line.
-
3. Propagation and Storage:
- 3.1. The recipient may not propagate the cells, unless material is marked as Propagation Model, Cell Bank or Master Cell Bank; then recipient may propagate and store the cells for long-term use.
-
4. For all determinations of luminescence or other assay-related activity of this material and its derivatives, recipient must either:
- 4.1. Use the detection systems specified in the protocol or data report and purchased from Promega for all luminescence assays when using this material; or
- 4.2. Contact Promega to obtain a license for use of this material with reagents other than a Bio-Glo™ branded luciferase reagent, including but not limited to:
- 4.2.1 Bio-Glo™, Bio-Glo-NL™, Bio-Glo-NB™ TCK, Bio-Glo-NB™ VLP, Bio-Glo-NB™ Lytic
-
5. For uses of this material for energy transfer (such as bioluminescence resonance energy transfer), recipient must:
- 5.1. Use NanoBRET™-branded luminescent assay reagents (LARs; e.g., NanoBRET™ Nano-Glo® Substrate), Intracellular TE Nano-Glo® Substrate/Inhibitor or Intracellular TE Nano-Glo® Vivazine™/Inhibitor for all determinations of luminescent activity by this material and its derivatives; and
- 5.2. Use NanoBRET™-branded energy acceptors (e.g., NanoBRET™ tracers, NanoBRET™ dyes, BRET-optimized HaloTag® ligands) for all determinations of energy transfer activity by this material and its derivatives; or
- 5.3. Contact Promega to obtain a license for the use of the material and its derivatives for energy transfer assays to energy acceptors not manufactured by Promega. No license is needed if the energy transfer acceptor is a genetically encoded autofluorescent protein.
-
6. For uses of HaloTag® Technology in this material, recipient must either:
- 6.1. Use Promega HaloTag® ligands, which can be modified or linked to Promega or customer-supplied moieties; or
- 6.2. Contact Promega to obtain a license if Promega HaloTag® ligands are not to be used.
-
7. Disclaimer and Governing Law:
- 7.1. PROMEGA MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING FOR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH REGARD TO THE MATERIAL. The terms of this label license shall be governed under the laws of the State of Wisconsin, USA.
What's in the box?
| Item | Part # | Size |
|---|---|---|
Human T Cell (CD8+), TDCC Qualified |
CS3055B01 | 1 × 0.5ml |
Promega Cell Line Limited Use Label License (LULL)
BY USE OF THIS MATERIAL, RECIPIENT AGREES TO BE BOUND BY THE TERMS OF THIS LIMITED USE LABEL LICENSE.
-
1. Usage and Transfer Restrictions:
- 1.1. Recipient may use this material for research use only, which includes but is not limited to drug discovery and development, quality assurance testing and product release assays. No transfer, resale or commercial use of this material is allowed without the written consent of Promega Corporation.
- 1.2. “Commercial use” means any and all uses of this material or derivatives by recipient for monetary or other consideration, including product manufacture, providing a service, information or data to unaffiliated third parties and resale of the material for any use.
-
2. Modification Restrictions:
- 2.1. Recipient shall have no right to modify, derivatize or otherwise create variations of the nucleotide sequence of any proprietary gene, including but not limited to luciferase, NanoBiT® technology (e.g., HiBiT), HaloTag® technology or genes stably transfected within the cells.
- 2.2. Recipient shall have no right to genetically engineer or otherwise modify the cell line.
-
3. Propagation and Storage:
- 3.1. The recipient may not propagate the cells, unless material is marked as Propagation Model, Cell Bank or Master Cell Bank; then recipient may propagate and store the cells for long-term use.
-
4. For all determinations of luminescence or other assay-related activity of this material and its derivatives, recipient must either:
- 4.1. Use the detection systems specified in the protocol or data report and purchased from Promega for all luminescence assays when using this material; or
- 4.2. Contact Promega to obtain a license for use of this material with reagents other than a Bio-Glo™ branded luciferase reagent, including but not limited to:
- 4.2.1 Bio-Glo™, Bio-Glo-NL™, Bio-Glo-NB™ TCK, Bio-Glo-NB™ VLP, Bio-Glo-NB™ Lytic
-
5. For uses of this material for energy transfer (such as bioluminescence resonance energy transfer), recipient must:
- 5.1. Use NanoBRET™-branded luminescent assay reagents (LARs; e.g., NanoBRET™ Nano-Glo® Substrate), Intracellular TE Nano-Glo® Substrate/Inhibitor or Intracellular TE Nano-Glo® Vivazine™/Inhibitor for all determinations of luminescent activity by this material and its derivatives; and
- 5.2. Use NanoBRET™-branded energy acceptors (e.g., NanoBRET™ tracers, NanoBRET™ dyes, BRET-optimized HaloTag® ligands) for all determinations of energy transfer activity by this material and its derivatives; or
- 5.3. Contact Promega to obtain a license for the use of the material and its derivatives for energy transfer assays to energy acceptors not manufactured by Promega. No license is needed if the energy transfer acceptor is a genetically encoded autofluorescent protein.
-
6. For uses of HaloTag® Technology in this material, recipient must either:
- 6.1. Use Promega HaloTag® ligands, which can be modified or linked to Promega or customer-supplied moieties; or
- 6.2. Contact Promega to obtain a license if Promega HaloTag® ligands are not to be used.
-
7. Disclaimer and Governing Law:
- 7.1. PROMEGA MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING FOR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH REGARD TO THE MATERIAL. The terms of this label license shall be governed under the laws of the State of Wisconsin, USA.
What's in the box?
| Item | Part # | Size |
|---|---|---|
Human T Cell (CD8+), TDCC Qualified |
CS3055B01 | 5 × 0.5ml |
Promega Cell Line Limited Use Label License (LULL)
BY USE OF THIS MATERIAL, RECIPIENT AGREES TO BE BOUND BY THE TERMS OF THIS LIMITED USE LABEL LICENSE.
-
1. Usage and Transfer Restrictions:
- 1.1. Recipient may use this material for research use only, which includes but is not limited to drug discovery and development, quality assurance testing and product release assays. No transfer, resale or commercial use of this material is allowed without the written consent of Promega Corporation.
- 1.2. “Commercial use” means any and all uses of this material or derivatives by recipient for monetary or other consideration, including product manufacture, providing a service, information or data to unaffiliated third parties and resale of the material for any use.
-
2. Modification Restrictions:
- 2.1. Recipient shall have no right to modify, derivatize or otherwise create variations of the nucleotide sequence of any proprietary gene, including but not limited to luciferase, NanoBiT® technology (e.g., HiBiT), HaloTag® technology or genes stably transfected within the cells.
- 2.2. Recipient shall have no right to genetically engineer or otherwise modify the cell line.
-
3. Propagation and Storage:
- 3.1. The recipient may not propagate the cells, unless material is marked as Propagation Model, Cell Bank or Master Cell Bank; then recipient may propagate and store the cells for long-term use.
-
4. For all determinations of luminescence or other assay-related activity of this material and its derivatives, recipient must either:
- 4.1. Use the detection systems specified in the protocol or data report and purchased from Promega for all luminescence assays when using this material; or
- 4.2. Contact Promega to obtain a license for use of this material with reagents other than a Bio-Glo™ branded luciferase reagent, including but not limited to:
- 4.2.1 Bio-Glo™, Bio-Glo-NL™, Bio-Glo-NB™ TCK, Bio-Glo-NB™ VLP, Bio-Glo-NB™ Lytic
-
5. For uses of this material for energy transfer (such as bioluminescence resonance energy transfer), recipient must:
- 5.1. Use NanoBRET™-branded luminescent assay reagents (LARs; e.g., NanoBRET™ Nano-Glo® Substrate), Intracellular TE Nano-Glo® Substrate/Inhibitor or Intracellular TE Nano-Glo® Vivazine™/Inhibitor for all determinations of luminescent activity by this material and its derivatives; and
- 5.2. Use NanoBRET™-branded energy acceptors (e.g., NanoBRET™ tracers, NanoBRET™ dyes, BRET-optimized HaloTag® ligands) for all determinations of energy transfer activity by this material and its derivatives; or
- 5.3. Contact Promega to obtain a license for the use of the material and its derivatives for energy transfer assays to energy acceptors not manufactured by Promega. No license is needed if the energy transfer acceptor is a genetically encoded autofluorescent protein.
-
6. For uses of HaloTag® Technology in this material, recipient must either:
- 6.1. Use Promega HaloTag® ligands, which can be modified or linked to Promega or customer-supplied moieties; or
- 6.2. Contact Promega to obtain a license if Promega HaloTag® ligands are not to be used.
-
7. Disclaimer and Governing Law:
- 7.1. PROMEGA MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING FOR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH REGARD TO THE MATERIAL. The terms of this label license shall be governed under the laws of the State of Wisconsin, USA.
What's in the box?
| Item | Part # | Size |
|---|---|---|
Human PBMC, ADCC Qualified |
CS3055A01 | 1 × 1 each |
Promega Cell Line Limited Use Label License (LULL)
BY USE OF THIS MATERIAL, RECIPIENT AGREES TO BE BOUND BY THE TERMS OF THIS LIMITED USE LABEL LICENSE.
-
1. Usage and Transfer Restrictions:
- 1.1. Recipient may use this material for research use only, which includes but is not limited to drug discovery and development, quality assurance testing and product release assays. No transfer, resale or commercial use of this material is allowed without the written consent of Promega Corporation.
- 1.2. “Commercial use” means any and all uses of this material or derivatives by recipient for monetary or other consideration, including product manufacture, providing a service, information or data to unaffiliated third parties and resale of the material for any use.
-
2. Modification Restrictions:
- 2.1. Recipient shall have no right to modify, derivatize or otherwise create variations of the nucleotide sequence of any proprietary gene, including but not limited to luciferase, NanoBiT® technology (e.g., HiBiT), HaloTag® technology or genes stably transfected within the cells.
- 2.2. Recipient shall have no right to genetically engineer or otherwise modify the cell line.
-
3. Propagation and Storage:
- 3.1. The recipient may not propagate the cells, unless material is marked as Propagation Model, Cell Bank or Master Cell Bank; then recipient may propagate and store the cells for long-term use.
-
4. For all determinations of luminescence or other assay-related activity of this material and its derivatives, recipient must either:
- 4.1. Use the detection systems specified in the protocol or data report and purchased from Promega for all luminescence assays when using this material; or
- 4.2. Contact Promega to obtain a license for use of this material with reagents other than a Bio-Glo™ branded luciferase reagent, including but not limited to:
- 4.2.1 Bio-Glo™, Bio-Glo-NL™, Bio-Glo-NB™ TCK, Bio-Glo-NB™ VLP, Bio-Glo-NB™ Lytic
-
5. For uses of this material for energy transfer (such as bioluminescence resonance energy transfer), recipient must:
- 5.1. Use NanoBRET™-branded luminescent assay reagents (LARs; e.g., NanoBRET™ Nano-Glo® Substrate), Intracellular TE Nano-Glo® Substrate/Inhibitor or Intracellular TE Nano-Glo® Vivazine™/Inhibitor for all determinations of luminescent activity by this material and its derivatives; and
- 5.2. Use NanoBRET™-branded energy acceptors (e.g., NanoBRET™ tracers, NanoBRET™ dyes, BRET-optimized HaloTag® ligands) for all determinations of energy transfer activity by this material and its derivatives; or
- 5.3. Contact Promega to obtain a license for the use of the material and its derivatives for energy transfer assays to energy acceptors not manufactured by Promega. No license is needed if the energy transfer acceptor is a genetically encoded autofluorescent protein.
-
6. For uses of HaloTag® Technology in this material, recipient must either:
- 6.1. Use Promega HaloTag® ligands, which can be modified or linked to Promega or customer-supplied moieties; or
- 6.2. Contact Promega to obtain a license if Promega HaloTag® ligands are not to be used.
-
7. Disclaimer and Governing Law:
- 7.1. PROMEGA MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING FOR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH REGARD TO THE MATERIAL. The terms of this label license shall be governed under the laws of the State of Wisconsin, USA.
What's in the box?
| Item | Part # | Size |
|---|---|---|
Human PBMC, ADCC Qualified |
CS3055A01 | 5 × 1 each |
Resources
Featured Resource
Cell Therapy: Decoding CAR-T
CAR-T cellular immunotherapy is a groundbreaking cancer treatment that uses genetically reprogrammed T-cells to target and destroy cancer cells. Watch this video to learn more about CAR-T cellular immunotherapy and how it is revolutionizing the world of cancer treatment.

Other Resources
Related Products
Similar Products
ADCC Reporter Bioassay, V Variant
Measure potency and stability of antibodies mediating ADCC through the high-affinity human FcγRIIIa-V receptor.
G7015, G7014, G7010, G7016, G7013, G7018, G7102, GA1130
T Cell Activation Bioassay (TCRαβ-KO)
Measure the potency of transgenic TCR constructs to activate T cells without the constraints of endogenous TCR expression.
GA1172, GA1162, GA1182, GA1210, GA1220, GA1230
FcγRIIa-H ADCP Bioassay
Characterize the Fc effector activity of antibodies that bind and activate the human FcγRIIa-H receptor.
G9901, G9991, G9995, G9871
IL-2 Bioassay
A bioluminescent cell-based assay designed to measure IL-2 stimulation or inhibition.
JA2201, JA2205, J2952
Frequently Used With
ADCP Reporter Bioassay (THP-1)
Measure the potency and stability of antibodies and other biologics with Fc domains that bind and activate FcγRs.
JA9411, JA9415, GA1272, GA6020
Bio-Glo-NB™ TCK Luciferase Assay Systems
A highly sensitive, robust and homogeneous reagent for the detection of cell killing in Promega TCK Bioassays.
JB1001, JB1002, JB1003
Lumit® FcγR Binding Immunoassays
Novel homogeneous competition assays to measure the interaction between human Fc receptors and antibodies or Fc fusion proteins.
CS3041A01, CS3041A02, CS3041A03, CS3041A04, CS3041A05
Bio-Glo-NB™ Lytic Luciferase Assay Systems
A highly sensitive, robust and homogeneous reagent for the detection of cell killing in ADCP-MoA TCK Bioassays.
JB1101, JB1102, JB1103



