RNA Purification
An overview of RNA extraction with details for both manual and automated purification methods.
Introduction
RNA, or ribonucleic acid, plays a crucial role in various biological processes and is central to the fundamental principles of molecular biology. Each type of RNA serves specific functions that are vital for the life of a cell and the organism.
Fundamental Roles of RNA
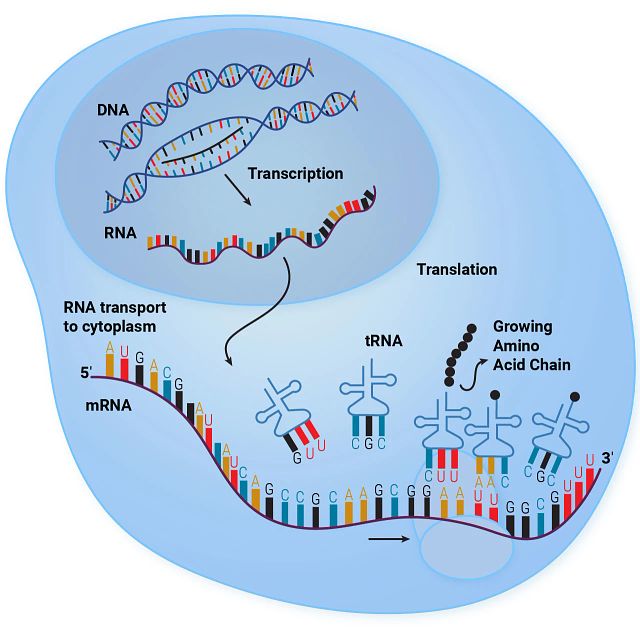
- Gene Expression Regulation: RNA is essential in the flow of genetic information from DNA to proteins, a central dogma of molecular biology. mRNA (messenger RNA) serves as the template for translating genetic information into proteins, directly impacting cellular structure and function.
- Protein Synthesis: tRNA (transfer RNA) and rRNA (ribosomal RNA) are pivotal in the process of protein synthesis. tRNA brings amino acids to the ribosome, where rRNA helps catalyze the assembly of amino acids into protein chains.
- Genetic Regulation and RNA Interference: Small RNAs like miRNA (microRNA) and siRNA (small interfering RNA) are involved in RNA interference, a regulatory mechanism used by cells to regulate the genes post-transcriptionally. This involves silencing specific mRNA molecules, thus controlling the proteins that get produced.
RNA purification is a crucial process in molecular biology, and is necessary for applications such as transcriptome analysis, RT-PCR and RNA sequencing. High-quality RNA is essential for reliable results in any downstream application, making the choice of an appropriate RNA isolation system critical. This guide provides an overview of RNA extraction, purification and quantitation methods, and introduces our innovative solutions tailored to enhance productivity and reliability in RNA purification.
RNA isolation is a specific subset of RNA purification processes aimed at extracting RNA that represents the whole transcriptome, including all RNA species present in a cell or tissue sample. This includes messenger RNA (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA) and other non-coding RNAs. RNA isolation is critical for applications such as RNA-Seq, which requires a comprehensive view of the transcriptome.
The goal for total RNA isolation is to obtain a representative snapshot of all RNA present in the original sample. This RNA can then be used for comprehensive analysis and insights into gene expression patterns, regulation and more. Our specialized kits and instruments facilitate this process with high reproducibility and efficiency, making them ideal for high-throughput applications and critical research.
Types of RNA
RNA can be categorized into various subtypes based on size, structure or function. Each RNA subtype plays distinct roles in cellular processes, and their study helps elucidate the complexities of gene expression and regulation across different biological contexts and conditions. Each type of RNA serves specific functions that are vital for the life of a cell and the organism.
| Type of RNA | Physical Description | Key Functions |
|---|---|---|
| Total RNA | Various sizes, single-stranded | Provides a comprehensive snapshot of all cellular RNA; used in broad research and diagnostic applications. |
| Messenger RNA (mRNA) | Single-stranded, variable length with poly(A) tail | Translates genetic information from DNA to proteins; essential for studying gene expression. |
| Non-Coding RNA (ncRNA) | Single-stranded, sizes vary from very long to circular structures | Involved in gene regulation, epigenetic regulation, and cellular structure; crucial for understanding gene expression control. |
| MicroRNA (miRNA) and Small RNA (sRNA) | Single-stranded, approximately 22 nucleotides long | Regulates gene expression post-transcriptionally; important in development, cell death, and disease progression. |
Total RNA
Total RNA comprises all RNA types present within a cell, including mRNA, rRNA, tRNA, miRNA, and various other non-coding RNAs. While total RNA is mostly single stranded, it can form many structures such as stem loops, and can include a wide range of molecules, both small and large that fulfill the diverse roles and functions of RNA within the cell.
Extracting and studying total RNA provides a comprehensive snapshot of the transcriptomic profile of a cell at a specific time including mRNA, rRNA, tRNA, and non-coding RNAs. This is crucial for broad-spectrum studies aimed at analyzing overall gene expression and assessing the impacts of various treatments on cellular RNA. This makes total RNA particularly valuable for both general research and diagnostic applications.
There are several downstream applications that are used to assess the quality and quantity of total RNA, common assays include quantitative RT-PCR (RT-qPCR), digital PCR (RT-dPCR) Northern blotting and RNA-Seq are employed.
Messenger RNA (mRNA)
Messenger RNA (mRNA) is a single-stranded molecule that serves as the crucial intermediary between DNA and the ribosome, carrying encoded genetic information that specifies the amino acid sequence of proteins during gene expression. The study of mRNA is fundamental in exploring gene expression patterns across various contexts such as gene regulation, disease progression, and developmental stages. Isolating mRNA is particularly essential for synthesizing complementary DNA (cDNA), which is then used in cloning and quantitative PCR (qPCR) to measure gene expression levels accurately.
Purification of mRNA can be expedited by exploiting the poly(A) tail found at the 3' end of most eukaryotic mRNAs. Magnetic beads or columns coated with oligo(dT) sequences are commonly used to selectively bind poly(A)+ mRNA, effectively separating it from other RNA species. This type of isolation is particularly useful for applications such as cDNA synthesis and transcriptome analysis that are focused on gene expression. Assays that are often used to analyze mRNA include RT-PCR, microarray analysis, and RNA sequencing (RNA-Seq).
Non-Coding RNA (ncRNA)
Non-coding RNA (ncRNA) is an expansive category of RNA that encompasses all RNA molecules that are not translated into proteins. This includes long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and small nucleolar RNAs (snoRNAs), among others.
Non-coding RNAs play crucial roles in various cellular processes including gene regulation, RNA processing, RNA modifications, maintaining cellular structure and modulating gene expression levels. Isolating ncRNAs is essential for understanding their diverse functions in gene expression control, epigenetic regulation, and their potential applications in diagnostics and therapeutic strategies.
To study ncRNAs, researchers commonly employ techniques such as RNA sequencing (RNA-Seq), hybridization-based approaches, and RNA immunoprecipitation sequencing (RIP-seq). ncRNAs are typically single-stranded molecules and can vary significantly in size; for instance, lncRNAs can be very long, whereas circRNAs form closed loop structures without free ends.
MicroRNA (miRNA) and Small RNA (siRNA, snRNA, etc.)
MicroRNAs (miRNAs) are a specific subset of small ncRNAs. They are single stranded molecules that are approximately 22 nucleotides in length. miRNAs play an important role in post-transcriptional regulation of gene expression and are integral to various biological processes including developmental timing, cell death, cell proliferation and the pathogenesis of cancer.
Isolating these small RNA molecules involves techniques that can effectively capture RNA fragments typically shorter than 200 nucleotides. Specialized kits are available that enhance the recovery of these small RNAs, which are important for studies on RNA interference and regulatory networks.
The short length of miRNA often necessitates the use of specific adapters to facilitate their amplification and sequencing in laboratory studies. Common techniques used to analyze miRNAs include Northern blotting, quantitative RT-PCR (qRT-PCR), and RNA sequencing (RNA-Seq) that is specifically adapted for small RNAs.
New small RNAs are continually being discovered, and our understanding of their roles in cellular processes is constantly evolving. However, several categories of small RNAs have been well characterized, each with known functions and mechanisms of action. Table 2 summarizes the different categories of small RNAs.
Table 2. Small RNA types, function and their applications
| Small RNA Types | Function | Applications |
|---|---|---|
| MicroRNAs (miRNAs) | Regulate gene expression by binding to complementary sequences on target mRNAs, typically resulting in gene silencing through translational repression or mRNA degradation. | Widely studied for roles in development, cancer, and other diseases. Used as biomarkers and potential therapeutic targets. |
| Small Interfering RNAs (siRNAs) | Involved in the RNA interference (RNAi) pathway, mediating the degradation of specific mRNA targets with perfect or near-perfect complementarity. | Used in research to silence gene expression; therapeutically explored for treating viral infections, genetic disorders, and cancers. |
| Piwi-interacting RNAs (piRNAs) | Complex with Piwi proteins and primarily silence transposable elements in germ cells, protecting genomic integrity. | Essential for studying fertility, germ cell development; potential factors in cancer biology. |
| Small Nuclear RNAs (snRNAs) | Critical components of the spliceosome responsible for pre-mRNA splicing, aiding in the processing of precursor mRNA into mature mRNA. | Vital for understanding gene expression regulation and splicing errors leading to disease. |
| Small Nucleolar RNAs (snoRNAs)) | Guide chemical modifications of other RNAs, including rRNAs and snRNAs, involved in methylation and pseudouridylation. | Studied for roles in rRNA modification and processing, increasingly implicated in cancer. |
Small RNA Resources
For researchers and professionals looking for a more comprehensive understanding or specific small RNAs, several databases and resources can provide extensive information:
- miRbase https://www.mirbase.org/ : Provides searchable databases of published miRNA sequences and annotation.
- RNAcentral https://rnacentral.org/ : Offers an integrated database of ncRNA sequences.
- Each of these databases is updated regularly with new findings from ongoing research, contributing to the dynamic understanding of small RNAs and their multifaceted roles in cellular biology and disease.
Additional Small RNA Resources
- Brancato, V. et al. (2022) News from around the RNA world: new avenues in RNA biology, biotechnology and therapeutics from the 2022 SIBBM meeting. Biol Open. 11, bio059597.
- Gingeras, T. (2023) Current frontiers in RNA research. Front. RNA Res.1:1152146.
Ribosomal RNA (rRNA)
Ribosomal RNA (rRNA) is a fundamental component of the ribosome and is crucial for protein synthesis in all living cells. It makes up about 80% of the total RNA in a cell and is characterized by its ability to form complex secondary structures. rRNA is extensively used as a control in various experimental setups to ensure equal RNA input during comparative analyses, aiding in the normalization of data.
The isolation of rRNA is particularly important for studies focused on understanding the intricacies of ribosome function and structure. In RNA sequencing (RNA-Seq) preparations, rRNA is often selectively depleted to enhance the visibility and sequencing depth of other RNA types, such as mRNA and non-coding RNA, which are present in much lower quantities. This depletion is crucial for reducing sequencing redundancy and improving the cost-effectiveness of sequencing experiments.
Metagenomics and species identification using ribosomal RNA (rRNA) are powerful approaches in microbiology that leverage the unique sequences of rRNA genes to classify and study microbial communities without the need for culturing. This methodology is especially useful in environmental and clinical settings where diverse microbial populations exist that are difficult to culture using standard laboratory techniques.
rRNA genes, particularly the 16S rRNA in prokaryotes and the 18S rRNA in eukaryotes, contain regions that are highly conserved across all species, as well as regions that vary significantly. The conserved regions allow for the binding of universal primers during PCR amplification, and the variable regions provide specific sequence differences that can be used to distinguish between species and sometimes even strains within a species.
Short-interfering (siRNA)
Small interfering RNA (siRNA), also known as short interfering RNA or silencing RNA, is a class of double-stranded RNA molecules, typically about 20–25 nucleotides long. is a critical component of the RNA interference (RNAi) pathway within cells, serving as a powerful tool for precise gene silencing. These short, double-stranded RNA molecules are designed to match specific sequences on messenger RNA (mRNA), leading to targeted mRNA degradation and thus preventing the translation of specific proteins. This mechanism is vital for regulating gene expression and can be exploited to study gene function or modulate disease processes.
siRNAs include a 2-nucleotide overhangs at each 3' end. These overhangs are important for the proper incorporation of siRNA into the RNA-induced silencing complex (RISC), which is responsible for the gene-silencing activity. The double-stranded structure of siRNA is essential for its function, as it allows one strand (the guide strand) to be loaded into RISC and direct the complex to the complementary mRNA target for cleavage.
The study of siRNA has profound implications in understanding gene regulation and exploring its therapeutic potential. By selectively silencing genes, siRNA allows researchers to dissect gene functions and investigate how disrupting specific mRNA transcripts affects cellular processes and disease development, such as in cancer or viral infections. This targeted approach helps to pinpoint the roles of genes in complex cellular pathways and offers a promising avenue for developing gene-specific therapies.
Isolating and studying siRNA is important for designing effective gene silencing strategies that are specific, efficient and cause minimal off-target effects. The use of siRNA in research and therapy hinges on the ability to accurately assess and control its interaction with its mRNA targets, ensuring that the intended gene silencing occurs without unintended consequences.
Assays that are commonly used to study siRNA include RNA Sequencing (RNA-Seq) to observe the global effects of siRNA on gene expression, verifying the specificity and extent of gene silencing; Northern Blotting helps in detecting and measuring the expression levels of siRNA itself or its target mRNA to evaluate the efficiency of the siRNA intervention; and Reporter Gene Assays can be crucial for validating the function of siRNA by linking the siRNA sequence to a reporter gene that can visually or quantitatively indicate successful gene silencing.
Transfer RNA (tRNA)
Transfer RNA (tRNA) is an important component of the protein synthesis machinery within cells and functions as the adaptor molecule that translates messenger RNA (mRNA) sequences into proteins. During translation, tRNAs recognize specific codons on the mRNA strand and bring the corresponding amino acids to the ribosome, where proteins are synthesized. This makes tRNA essential for the translation process.
The study of tRNA has significant implications in understanding protein synthesis and how its modifications can influence cellular responses to stress and contribute to diseases such as cancer. For this reason, isolating tRNA can be pivotal for detailed studies on protein synthesis dynamics and the functional impacts of tRNA modifications under various physiological and pathological conditions.
Commonly employed assays to study tRNA include modified RNA sequencing and northern blotting, which can analyze and quantify tRNA modifications and expression. Physically, tRNAs are small, single-stranded RNA molecules that fold into a characteristic cloverleaf structure, which includes an anticodon loop that matches specific mRNA codons and an opposite end that attaches to a specific amino acid, facilitating its incorporation into the growing polypeptide chain.
Interrogating RNA
RNA interrogation is fundamental in molecular biology and medical research because RNA serves as a crucial mediator and regulator in the flow of genetic information from DNA to proteins. Studying RNA helps scientists understand how cells function under normal conditions and what changes occur when things go wrong, such as in diseases. Here are several reasons why RNA is so central to biological research:
- Central Role in Gene Expression
RNA is directly involved in gene expression, acting as the intermediary between the genetic instructions in DNA and the production of proteins. Messenger RNA (mRNA), for instance, carries the genetic blueprint from DNA to the ribosome, where proteins are synthesized. By studying mRNA, researchers can directly assess which genes are active in a cell at any given time and under specific conditions. - Regulation of Gene Expression
RNA molecules, particularly non-coding RNAs like microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), play critical roles in regulating gene expression. They can enhance or suppress the production of specific proteins by interacting with other RNAs or directly with DNA. By understanding these interactions, researchers can decipher complex regulatory networks that maintain cellular functions and adapt to new conditions. - Disease Mechanisms
Many diseases, including cancers, genetic disorders and infections, involve changes in gene expression and RNA function. Abnormal levels of certain RNAs or mutations in RNA molecules can lead to dysfunctional proteins or inappropriate cellular responses. For example, some cancers are driven by mutations that affect mRNA, leading to the production of oncogenic proteins. Other diseases, like some forms of muscular dystrophy, are associated with misregulated splicing of mRNA. - Developmental Biology
During development, precise control of gene expression is critical. RNA plays a key role in this process, with specific RNAs necessary at different stages of cell differentiation and organ development. Errors in RNA-based regulation during development can lead to developmental disorders and congenital abnormalities. - Response to Environmental Stress
Cells respond to environmental stresses—such as changes in temperature, toxins or nutrient availability—by altering gene expression. These responses are often mediated by changes in RNA, including both mRNA and non-coding RNAs. Studying these responses can reveal mechanisms of cellular adaptation, survival and stress-related diseases. - Therapeutic Targets and Tools
RNAs are not only biomarkers for various diseases but also targets for therapeutic interventions. RNA-based drugs, including small interfering RNAs (siRNAs) and antisense oligonucleotides, are being developed to silence specific genes implicated in diseases. Moreover, the recent advances in mRNA vaccines highlight the therapeutic potential of RNA. - Evolutionary Insights
Comparative studies of RNA across different organisms can provide insights into evolutionary processes. Ribosomal RNA (rRNA), due to its evolutionary conservation, is extensively used in phylogenetic studies to trace the evolutionary relationships among organisms.
RNA Regulation
RNA regulation is a critical aspect of cellular control mechanisms, influencing how genes are expressed and how cells respond to their environment. The regulation of RNA impacts all stages of an RNA molecule’s life cycle, from its synthesis to its processing, transport, translation and eventual degradation. Understanding and manipulating these regulatory processes is important for both basic science and medical applications. Here’s a breakdown of how RNAs are regulated and the reasons for wanting to control these processes:
Mechanisms of RNA Regulation
- Transcriptional Control:
- Promoter Regulation: The activity of promoters, where RNA polymerase binds to initiate transcription, can be modulated by transcription factors, which either enhance or suppress transcription initiation.
- Epigenetic Modifications: DNA methylation and histone modifications can affect transcription indirectly by changing the accessibility of DNA to transcriptional machinery.
- RNA Processing:
- Alternative Splicing: Pre-mRNA can be spliced in different ways to produce multiple mRNA variants from a single gene, allowing for a diverse protein output.
- 5' Capping and Polyadenylation: Modifications such as the addition of a 5' cap and a poly-A tail are crucial for mRNA stability and translation efficiency.
- Export and Localization:
- Nuclear Export: Only properly processed mRNAs are exported from the nucleus to the cytoplasm where translation occurs.
- Localization: mRNA localization in different regions of the cell can influence where proteins are synthesized, affecting cellular architecture and signaling pathways.
- Translation Regulation:
- Ribosome Binding: The efficiency of ribosome binding to the mRNA, influenced by the mRNA’s secondary structure and initiation factors, regulates translation initiation.
- Regulatory Proteins and miRNAs: Proteins and miRNAs can bind to mRNA to prevent ribosome access, reducing or halting translation.
- RNA Degradation:
- Turnover and Stability: Specific sequences within RNA molecules, such as AU-rich elements, dictate their stability and how quickly they are degraded in the cell.
- Nonsense-Mediated Decay: mRNAs with premature stop codons are recognized and rapidly degraded, preventing the production of truncated or malfunctioning proteins.
Reasons for Regulating RNA
- Cellular Function and Homeostasis:
- Regulating RNA is fundamental for cells to maintain homeostasis and respond adaptively to internal and external changes. For instance, cells might alter the levels of certain mRNAs to adjust their metabolic processes in response to stress.
- Development and Differentiation:
- During development, precise RNA regulation ensures that genes are expressed at the correct times and in the correct cells, guiding the development of tissues and organs.
- Disease Prevention and Treatment:
- Many diseases, including cancers and genetic disorders, are associated with misregulated RNA expression. Understanding RNA regulation can lead to targeted therapies, such as using antisense oligonucleotides to modify splicing patterns or miRNAs to suppress oncogene expression.
- Research and Biotechnology Applications:
- In research, artificially regulating RNA can help elucidate the function of specific genes. In biotechnology, RNA regulation is exploited to enhance yields of therapeutic proteins or to engineer cells with novel functions.
- Therapeutic Strategies:
- RNA-based therapies, including siRNA and mRNA vaccines, rely on delivering or modulating RNA in cells. Effective RNA regulation enhances the success and specificity of these treatments.
By studying RNA, researchers can gain insights into the fundamental processes that drive cellular function and organism development, understand pathological alterations that lead to disease and develop RNA-based technologies and treatments. The regulation of RNA is integral to almost every aspect of cell biology and is increasingly becoming a focus for therapeutic intervention. By manipulating RNA levels and activities, scientists can not only uncover fundamental biological mechanisms but also develop new strategies for treating complex diseases.
RNA Extraction and Purification Basics
RNA extraction involves several key steps that may vary slightly depending on the starting material and the specific protocol used. However, the general principles are similar and aim at efficiently isolating RNA while preventing degradation.
Key Steps in Genomic RNA Isolation
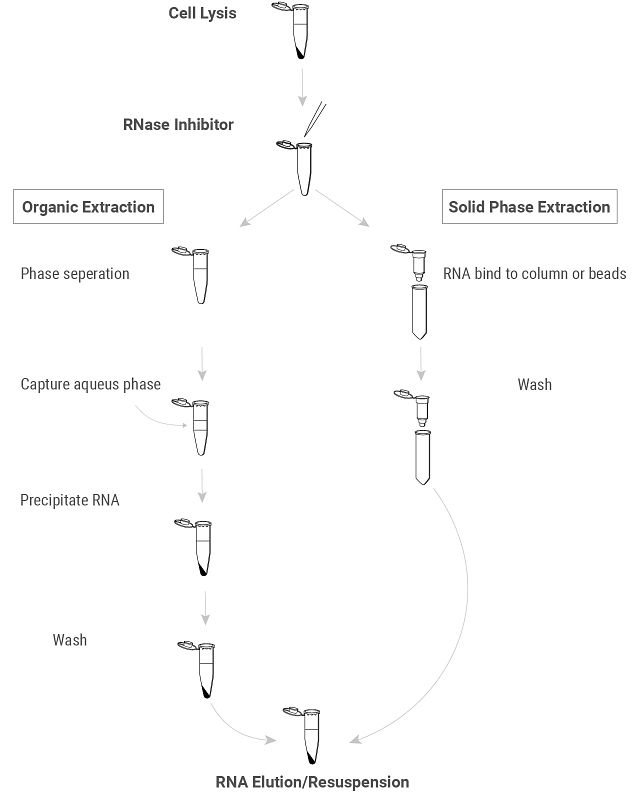
Extraction
- Sample Preparation:
- Depending on the sample type (e.g., blood, tissue, cells), preparation may involve homogenization or lysis under conditions that preserve RNA integrity and minimize RNAse activity.
- Cell Lysis:
- This can be achieved using physical methods (e.g., bead beating), chemical lysis buffers that include RNase inhibitors, or a combination of both. The goal is to disrupt the cell membrane and release all cellular contents, including RNA, into the solution.
- Physical Methods: Homogenization, beating or sonication.
- Chemical Methods: Utilizing detergents or chaotropic agents.
- Enzymatic Methods: Treatment with enzymes such as lysozyme or zymolyase to digest cell walls
- This can be achieved using physical methods (e.g., bead beating), chemical lysis buffers that include RNase inhibitors, or a combination of both. The goal is to disrupt the cell membrane and release all cellular contents, including RNA, into the solution.
- RNase Inhibition:
- RNase inhibitors are crucial from the beginning of the process to prevent the degradation of RNA by ubiquitous RNases. RNase inhibitors are often added during lysis to protect RNA from enzymatic degradation.
Purification
- Phase Separation/RNA Binding:
- There are two different approaches to separating RNA from other cellular components:
- Organic Purification (traditional): Some protocols, particularly traditional ones, use phenol-chloroform to separate proteins from nucleic acids. After this phase separation, RNA is precipitated using alcohol (e.g., ethanol or isopropanol) to concentrate it for further purification.
- Solid-Phase Extraction (Modern, Preferred): In many modern workflows, RNA binds directly to a silica membrane or magnetic beads in high-salt conditions, which selectively captures RNA and bypasses the need for organic solvents. This method provides a faster, cleaner process.
- There are two different approaches to separating RNA from other cellular components:
- Washing:
- Ethanol or isopropanol washes help remove impurities, ensuring that only RNA remains bound to the purification matrix.
- The bound RNA is washed one or more times to remove any remaining impurities such as proteins, DNA and low-molecular-weight metabolites.
- Resuspension/ Elution/
- Resuspension (organic protocols): The RNA pellet is resuspended in nuclease-free water or a minimal buffer.
- Elution (solid-phase protocols): The RNA is eluted from the column or beads using a low ionic strength solution, typically nuclease-free water or a minimal buffer.
Techniques Used in RNA Isolation
Column-Based Purification
Uses silica membranes or other affinity-based columns to bind RNA under certain ionic and pH conditions. The process typically involves lysing cells, applying the lysate to the column, washing off contaminants, and then eluting the RNA. It's fast, easy to use, and effective for obtaining high-purity RNA. Ideal for applications requiring high-quality RNA such as qRT-PCR and RNA sequencing.
Magnetic Bead-Based Purification
Utilizes magnetic particles or beads coated with substances that specifically bind RNA. Mixing these particles with the lysed sample allows the RNA to bind to the bead and then a magnet can be used to pull them aside, allowing for washing and subsequent RNA elution. This technique offers flexibility in sample volume and throughput, minimizes sample loss, and is amenable to automation. Used in high-throughput environments and where equipment for centrifugation is limited.
Phenol-Chloroform Extraction
This is a traditional chemical extraction method where phenol and chloroform are used to separate RNA from DNA and proteins. The sample is mixed with phenol-chloroform, centrifuged to separate phases, and RNA is recovered from the aqueous phase via ethanol precipitation. While it's labor-intensive and uses toxic chemicals, it effectively disrupts cells and denatures proteins, often resulting in very high yields of RNA. Suitable for tough, fibrous or highly proteinaceous samples.
Solid Phase Reversible Immobilization (SPRI)
Beads, coated with carboxyl groups, preferentially bind nucleic acids at certain salt concentrations. In conjunction with the presence of PEG, the beads are used to selectively capture and wash RNA. After washing, RNA is eluted with a low ionic strength solution. This method Provides scalability and is excellent for preparing samples for next-generation sequencing due to its ability to selectively bind different sizes of RNA or DNA by adjusting the PEG/salt ratio.
Salt-based methods
Salt (such as sodium acetate) is used to precipitate RNA from solution. RNA precipitates out of solution under high salt and low temperature. Because it’s simple and cost-effective, salt precipitation is often used as a secondary purification step following initial extraction to improve RNA purity.
Alcohol-based precipitation
Alcohol (typically isopropanol or ethanol) is added to the RNA solution to precipitate RNA. The mixture is centrifuged, and the RNA pellet is washed and solubilized. Alcohol precipitation is universally used for concentrating RNA and removing impurities and is compatible with most extraction methods. It is simple and effective for final RNA cleanup and concentration.
Other Popular methods
Guanidinium Thiocyanate-Phenol-Chloroform Extraction: An enhancement of the phenol-chloroform method, combining a powerful denaturing agent (guanidinium thiocyanate) with phenol and chloroform to improve RNA purity and yield. (1 )
TRIzol® Reagent Method: A proprietary variant of the guanidinium thiocyanate-phenol-chloroform extraction method, which effectively isolates RNA from cell or tissue samples. It’s crucial to process samples rapidly after addition of the reagent and to ensure complete phase separation during chloroform addition to prevent RNA degradation.
RNA Stabilization Solutions: These are not extraction methods per se, but are often used immediately after sample collection to preserve RNA integrity by inhibiting RNases.
Considerations for Working with RNA
Creating and RNase-Free Environment
RNA purification is a critical step in many biological research workflows, but it can be fraught with challenges that affect the quality and integrity of the RNA obtained. RNA is highly susceptible to degradation with RNases, ubiquitous enzymes that breaks RNA down into smaller components. RNase contamination can compromise the quality of samples and the accuracy of downstream applications. Creating an RNase-free environment for successful purification and downstream analysis of RNA.
Steps for Creating an RNase-Free Environment
- Prepare the Workspace: Thoroughly clean the workspace with RNase decontamination solutions or 0.1% DEPC (diethyl pyrocarbonate) treated water. Allow surfaces to air dry or wipe them down with RNase-free wipes.
- Use RNase-Free Consumables: Use RNase-free tubes, pipette tips, and other consumables. These are usually certified RNase-free by the manufacturer. Alternatively, you can treat plasticware with 0.1% DEPC-treated water followed by autoclaving.
- Wear Protective Gear: Always wear gloves and a lab coat to prevent contamination from skin, which can be a source of RNases. Change gloves frequently, especially if you touch surfaces that are not RNase-free.
- Use RNase-Free Reagents: Ensure all reagents used are RNase-free. This includes water, buffers, and any chemicals. Use DEPC-treated water or commercially available RNase-free water.
- Dedicated Equipment: Use equipment that is dedicated to RNA work only. If this is not possible, decontaminate equipment like pipettes by soaking them in 0.1% DEPC-treated water overnight followed by thorough rinsing with RNase-free water and autoclaving.
- Regular Decontamination: Periodically decontaminate the equipment and workspace. For example, UV irradiation can be used to sterilize surfaces and equipment.
- Avoid Talking and Breathing Directly: While handling RNA samples, avoid talking or breathing directly over open tubes to prevent contamination with RNases from saliva.
- Keep Samples Cold: RNA is more stable at lower temperatures. Keep samples and reagents on ice whenever possible and store RNA at –80°C for long-term storage.
- Use RNase Inhibitors: Consider using RNase inhibitors in your protocols to further protect RNA samples from degradation.
- Proper Disposal: Properly dispose of any waste that may be contaminated with RNases and avoid cross-contamination by keeping disposal areas separate from the RNA work areas.
General Tips for RNA Purification Across Sample Types
RNA purification is a critical step in many biological research workflows, but it can be fraught with challenges that affect the quality and integrity of the RNA obtained. Two major problem areas involve the lysis process without degrading RNA and the reduction of non-specific background in downstream applications.
Effective Lysis Without RNA Degradation
The process of lysing cells or tissues to release RNA must be done carefully to prevent RNA degradation. RNA molecules are particularly vulnerable because of ubiquitous RNases that can rapidly degrade RNA. Here are strategies to minimize RNA degradation during lysis:
- Use of RNase Inhibitors: Adding RNase inhibitors to lysis buffers is a common practice. These inhibitors can specifically bind to and inhibit RNases, thus protecting RNA from degradation during the lysis process. (See our RNase Inhibitors, featuring RNasin® Ribonuclease Inhibitor)
- Maintaining Cold Conditions: Performing all procedures on ice or at 4°C helps to slow down enzymatic activities, including those of RNases. Equipment and reagents should be pre-chilled, and samples should be kept on ice whenever possible.
- Immediate Processing: RNA should be extracted immediately after the tissue or cells are collected or lysed. If immediate processing is not possible, samples should be flash-frozen in liquid nitrogen and stored at –80°C or treated with RNA stabilization solutions to prevent degradation.
- Physical Methods of Lysis: Mechanical methods such as homogenization, bead beating, or sonication must be optimized to ensure complete cell disruption without heating the sample, which can increase RNase activity. For tough tissues, using liquid nitrogen to grind the samples into a fine powder before adding lysis buffer can be effective.
Reduction of Non-Specific Background
Non-specific background in RNA-based assays can obscure results and lead to misinterpretations. This background can arise from several sources during RNA purification:
- Contamination from Genomic DNA: DNA contamination can lead to non-specific signals, especially in RT-PCR assays. DNase treatment of purified RNA samples is crucial to digest any contaminating DNA without affecting the RNA.
- Carry-Over of Extraction Reagents: Phenol, ethanol, and salts from the extraction process can interfere with downstream applications if not completely removed during the RNA purification process. It’s important to thoroughly wash the RNA pellets and ensure complete removal of these substances during the purification steps.
- Incomplete Separation of RNA Types: When specific types of RNA (like mRNA) are needed, residual rRNAs and tRNAs can contribute to the background in sequencing and other sensitive assays. The use of oligo-dT columns for mRNA isolation or size-exclusion methods can help in enriching the desired RNA fractions.
- Chemical Modifications: Some RNA extraction methods can lead to chemically modified bases that create artifacts in high-throughput sequencing. Using reagents that minimize these modifications and ensuring proper handling are essential for maintaining RNA integrity.
Practical Tips:
- Optimize Protocols: Each type of sample might require specific conditions for optimal lysis and RNA recovery. It's often beneficial to experiment with different lysis methods and conditions.
- Quality Checks: Regularly check the quality and quantity of RNA using UV spectrophotometry, fluorometry, and gel electrophoresis. This helps in troubleshooting and refining the purification process.
- Standardize Procedures: Standardize RNA isolation protocols across samples to reduce variability and improve the comparability of experimental results.
Additional considerations for successful RNA purification
- RNA Integrity: The integrity of purified RNA is critical for applications like RNA sequencing. It is often assessed using an RNA Integrity Number (RIN) provided by instruments like the Agilent Bioanalyzer. Maintaining RNA integrity is perhaps the biggest challenge in RNA isolation. Measures to protect RNA from heat and enzymatic degradation are crucial.
- Sample Heterogeneity: Different tissues and cell types can vary in their RNA content and composition, which may require optimization of lysis and purification conditions. One might address this by enriching for the target RNA before purifying the RNA (e.g., isolated WBC from whole blood and only purify RNA from the WBCs only).
- Quantification and Validation: After isolation, it is essential to quantify RNA and assess its purity and integrity, typically using spectrophotometry and gel electrophoresis or advanced methods like capillary electrophoresis.
- Sample Storage: Purified RNA should be stored at –80°C in aliquots to avoid degradation resulting from multiple freeze-thaw cycles.
Sample Type Specific Considerations
Different sample types present unique challenges and requirements in RNA isolation procedures. Here are key considerations for various sample types:
Cell Culture
- Ease of RNA Extraction: Cultured cells are typically easier to work with than solid tissues because the cells can be lysed directly in the culture dish.
- Precautions: Be sure to wash cells thoroughly to remove media which might contain RNases.
- Low cell density or adherence of cells: Confirm that cells are at the optimal confluency before harvest. Use trypsin or other detachment methods for adherent cells and ensure complete cell lysis using appropriate lysis buffers.
Solid Tissues
- Physical disruption: Tissues require physical disruption, often through homogenization or grinding in liquid nitrogen, to ensure complete cell lysis.
- RNase Contamination: Tissues often contain higher levels of RNases; Homogenize tissues thoroughly using mechanical disruption tools like bead mills or tissue homogenizers. Homogenized samples should be rapidly processed and immediately stabilized with RNA preservation agents or frozen in liquid nitrogen.
- High probability for contamination: Consider including lipid removal steps, adjusting centrifugation speeds, using desalting and washing steps, employing cold extraction conditions, mechanical homogenization or enzymatic digestion to reduce the impact of protein, lipid, chemical or physical contaminants and to help lower the possibility of endogenous enzymatic activity.
Blood
- Special Reagents: Blood RNA extraction often requires specific reagents or kits to deal with the abundant proteins and RNases.
- Storage and Handling: Samples should be processed quickly after collection to prevent degradation of cellular and molecular components. If immediate processing is not possible, samples should be stored under conditions that preserve their integrity, such as freezing at –80°C.
- Sample enrichment: Depending on the specific research and diagnostic need, enriching for white blood cells (WBCs) or Plasma before RNA extraction may be required. Isolating these specific fractions can yield insights into immune functions, genetic disorders, infections and other systematic conditions.
- For WBCs: Blood samples can be fractionated using density gradient centrifugation or specific lysis buffers that selectively lyse red blood cells (RBCs), leaving WBCs intact.
- For Plasma: Centrifugation is used to separate plasma from cellular components of blood. Careful handling is required to prevent the lysis of blood cells, which can contaminate the plasma with intracellular components.
Plant Material
- Secondary Metabolites: Plants contain phenolics, sugars and other secondary metabolites that can interfere with RNA isolation and quality.
- Method Adaptation: Using reagents such as CTAB (cetyltrimethylammonium bromide) buffer that can help remove or bind these contaminants is necessary.
- Immediate processing: Quickly freezing plant tissues in liquid nitrogen upon collection and keeping them at low temperatures throughout the RNA extraction process will minimizes RNase activity.
- High content of polysaccharides and secondary metabolites like phenolics: Use extraction buffers containing high salt concentrations, detergents or organic solvents to remove contaminants. Including PVP (Polyvinylpyrrolidone) in the extraction buffer and CTAB-based methods are particularly effective techniques.
- Difficulty lysing the sample: Use mechanical disruption (e.g., homogenization or liquid nitrogen griding), to physically break down tough plant cell walls or chemical lysis techniques (e.g., buffer optimization) to help disrupt cell walls and inactivate RNases, can aid improve lysis efficiency.
- Potentially low abundance of RNA: Maximize extraction efficiency by optimizing extraction techniques like adjusting pH and ionic strength of the extraction buffer to enhance RNA solubility and stability. Consider increasing starting material to compensate for low RNA content. Concentration techniques such as precipitation enhancers or RNA concentration kits may also improve yields for low-abundance RNAs.
Microorganisms
- Cell Wall Lysis: Bacteria, yeast and fungi have tough cell walls that require mechanical disruption (e.g., bead beating) or enzymatic treatment (with lysozyme for bacteria and zymolyase for yeast) for effective lysis before extraction.
- Extraction Efficiency: The efficiency of RNA isolation can vary widely among different species due to differences in cell wall composition and structure.
Clinical Samples (e.g., Biopsies, Swabs)
- Sample Size and Quality: Rapid processing is essential as these samples are often limited in quantity and can degrade quickly.
- Stabilize using RNAlater: Stabilizing agents like RNAlater can be used immediately after sample collection to preserve RNA integrity until processing.
- Use a specialized Kit: Maximize your RNA yield and integrity by choosing an RNA extraction kit that is designed to handle small, degraded samples efficiently.
- Handle samples properly: How samples are collected, stored and shipped can impact quality and yield of RNA.
FFPE Samples (formalin-fixed, paraffin-embedded samples)
- Removal of paraffin embedding matrix: Paraffin must be thoroughly removed before proceeding; residual paraffin can inhibit enzymatic reactions (like PCR and Reverse transcription) that are critical downstream analyses. Ensure deparaffinization steps are meticulously optimized and followed. Use xylene, or xylene substitutes, followed by a series of ethanol washes to completely remove any residual paraffin. There are now fewer toxic solvents and detergents have been developed that perform similarly to xylene without the health and environmental risks.
- Quantity of sample material: Small samples (e.g. Fine Needle Aspirate samples) provide less material to work with and will potentially lead to lower yields of RNA.
- RNA crosslinking and fragmentation due to formalin fixation: Use specialized kits that include a deparaffinization step and conditions to reverse formalin-induced modifications. These kits often use proteinase K digestion to improve RNA recovery.
Environmental Samples (e.g., wastewater, treatment plant effluent, feces)
- Pre-treat Samples: These samples have the potential for a high level of contaminants and inhibitors along with variable RNA sources and concentration. Pre-treating samples to remove inhibitors is crucial. Differential centrifugation and filtration can help concentrate RNA from dilute samples like wastewater.
- Use specialized kits: Extraction kits designed for environmental samples with inhibitor removal technology can improve RNA yield and integrity.
- Use comprehensive cell lysis protocols: These samples may contain a highly diverse array of microorganisms with varying cell wall compositions that require different lysis methods. Using a combination of lysis methods that include physical (e.g., bead beating, sonication), chemical (e.g., lysis buffers with detergents) and enzymatic (e.g., lysozyme, proteinase K) approaches to ensure breakdown of all cell types. Optimizing lysis conditions for the specific sample type will help maximize RNA yield.
- Low biomass samples: Certain environmental samples, like high-altitude air or oligotrophic water, may contain very low biomass, making RNA extraction challenging and resulting in low yields. To improve yields, concentrate the samples using filtration, centrifugation or precipitation techniques or increase the volume or area.
Methods for Determining RNA Yield & Purity
Determining the yield and purity of the isolated RNA is critical for ensuring the quality and usability of the RNA in downstream applications.
The choice of method depends on several factors, including the nature of the RNA target, the required sensitivity and specificity, the context of the RNA within cells or tissues, and the available resources. Each method has its strengths and limitations, and often multiple approaches are used in combination.
Here are some commonly used methods for assessing RNA yield and purity:
Spectrophotometry (UV Absorbance)
A spectrophotometer measures the absorbance of ultraviolet light by RNA at 260nm (A260), where nucleic acids absorb light. RNA concentration is estimated based on this absorbance.
Purity is determined by calculating the ratio of absorbance at 260nm to 280nm (A260/A280). A ratio of ~2.0 is generally indicative of pure RNA that is free from protein contamination. The ratio of absorbance at 260nm to 230nm (A260/A230) is used to assess contamination by organic compounds or chaotropic salts, with ideal values above 1.8.
NanoDrop® spectrophotometers allow for the quantification of RNA using only 1–2µl of sample. The device measures UV absorbance at 260nm and calculates RNA concentration. NanoDrop® instruments also provides A260/A280 and A260/A230 ratios for assessing the purity of the RNA sample against protein and solvent contamination.
Challenges and Considerations:
- Sensitivity: Methods like spectrophotometry may require relatively high concentrations of RNA and can be susceptible to interference from contaminants that absorb at similar wavelengths. Fluorometry and capillary electrophoresis offer higher sensitivity and specificity.
- Sample Quality: RNA integrity is as important as its concentration. Degraded RNA may still give an adequate yield but will perform poorly in applications such as RNA sequencing or qRT-PCR.
- Contamination: Contamination from proteins, DNA or extraction chemicals can affect the A260/A280and A260/A230 ratios, misleading interpretations about RNA purity.
Fluorometry
Fluorometric methods involve the use of fluorescent dyes that specifically bind to RNA. The fluorescence intensity after binding to RNA provides a measure of RNA concentration.
Fluorometry is more sensitive than UV absorbance and can detect lower concentrations of RNA. It is also less susceptible to interference from contaminants that absorb UV light.
Fluorometry is typically very fast, making it an attractive method for quickly assessing RNA concentration and purity. However, the speed of the assay can also be a drawback if the sample cleanup process is not thorough. Residual contaminants that interfere with the fluorescent dye can lead to erroneous readings. Ensuring that RNA purification and cleanup processes are efficient and effective is essential to obtaining reliable results with fluorometry. Some fluorescent dyes used in fluorometry are not specific to RNA and can also bind to DNA or double-stranded RNA, leading to inaccurate measurements of RNA concentration. Using dyes that are highly specific for RNA can also help mitigate these issues.
It’s important to note that every fluorescent dye has a specific linear range within which it can accurately quantify the concentration of RNA. If the RNA concentration in your sample is too high or too low, it may fall outside of this linear range, leading to inaccurate quantification. In these cases, diluting the sample to bring the RNA concentration within the optimal range for the dye is necessary. This adjustment ensures that the fluorescence intensity measured correlates directly to the RNA concentration, providing accurate and reproducible results.
Agarose Gel Electrophoresis and Microfluidic Analysis
In traditional agarose gel analysis, RNA is loaded onto an agarose gel and subjected to an electric field, causing the RNA to migrate according to its size. Staining the gel with ethidium bromide or a similar dye allows you to visualize the RNA integrity and contamination (such as genomic DNA). The integrity of RNA can be accessed by examining the sharpness and intensity of the ribosomal RNA bands (28S and 18S rRNA in eukaryotes). The presence of a smeared pattern indicates degradation.
Microfluidic analysis uses microfluidic chips to perform capillary electrophoresis on small samples. Instruments like the Agilent Bioanalyzer or the TapeStation use microfluidic technology to separate RNA by size. RNA samples are loaded onto a chip with microfabricated channels, where they are subjected to an electric field. This method provides a digital output that visualizes the integrity of RNA samples, similar to traditional gel electrophoresis but with greater resolution and repeatability.
Microfluidic analysis is especially useful for assessing RNA integrity. It generates an RNA Integrity Number (RIN), which rates RNA from 1 (highly degraded) to 10 (intact). This quantitative assessment helps in evaluating the suitability of RNA for sensitive applications like RNA sequencing.
This method of analysis requires only minimal sample volumes, reduces sample-to-sample contamination, and provides faster and more consistent results than traditional gel electrophoresis. It's particularly useful in labs performing high-throughput RNA analysis and where precision is crucial.
RT-qPCR
Quantitative Polymerase Chain Reaction (qPCR), also known as Real-Time PCR, is used to amplify and simultaneously quantify a targeted sequence of interest. For RNA analysis, Reverse Transcription qPCR (RT-qPCR) first converts RNA into complementary DNA (cDNA) using reverse transcriptase, which is then amplified using qPCR.
RT-qPCR involves the use of specific primers that target a sequence of interest. The process is monitored in real-time using either fluorescent dyes that bind to the double-stranded DNA, or sequence-specific probes that fluoresce upon hybridization. The increase in fluorescence intensity is proportional to the amount of PCR product formed, which reflects the initial quantity of the target RNA.
RT-qPCR is widely used to quantify gene expression levels across different samples, making it a gold standard for validating gene expression seen in RNA-seq experiments. It is highly specific and sensitive, capable of detecting even small amounts of RNA. This method of analysis provides quantitative data that is essential for gene expression studies, pathogen quantification or any application where the precise quantification of RNA levels is necessary. RT-qPCR is highly reproducible, and a large number of samples can be analyzed quickly once the conditions are optimized.
Automated Systems for RNA Purification
As laboratories try to improve productivity for research, diagnostics and applied testing, the need has increased for easy-to-use, low- to moderate-throughput automation of purification processes. Automation eliminates the hands-on time of manual purification, improving laboratory efficiencies.
Cartridge-Based Systems
Traditionally, automation refers to the use of large, specialized and costly equipment that requires extensive training to operate and maintain. Promega has developed the Maxwell® Systems, which provide flexible, reliable, compact and easy-to-use alternatives to traditional automated systems.
The Maxwell® Systems are designed for efficient, automated purification from a wide range of sample types. Maxwell® Instruments are supplied with preprogrammed automated purification methods and can process up to 48 samples in as little as 30–40 minutes (depending on instrument, sample type and method). The purified concentrated DNA or RNA are high quality and high yield, making them compatible with many common downstream applications, including qPCR, ddPCR, genotyping, sequencing and NGS.

The Maxwell® RSC (left) and Maxwell® RSC 48 (right).
Plate-Based Systems
As laboratories seek more scalable and flexible automated solutions to meet their evolving needs, systems that use 96- or 24-well plate formats have become increasingly popular. These systems can handle a range of throughput demands from moderate to high, making them ideal for laboratories aiming to streamline their RNA purification processes without sacrificing precision.
Typically, plate-based systems process samples in one of two ways, by either using magnetic particles and a magnet on the instrument deck to capture and move nucleic acid from plate to plate or by using automated pipetting to move reagents and waste from the processing plate.
These systems produce RNA that is of high quality and purity and suitable for a wide array of downstream applications such as qPCR, ddPCR, genotyping, sequencing and next-generation sequencing (NGS). This makes plate-based systems an excellent choice for laboratories that require flexible, high-throughput options that can keep pace with the high demands of modern biological research, diagnostics and applied testing.
Lab Automation Implementation Support
Implementing automated nucleic acid purification or making changes to your high-throughput (HT) workflow can be complicated and time-consuming. There are also many barriers to success such as challenging samples types and maintaining desirable downstream results that can add to the stress, not to mention actually getting the robotic instrumentation to do what you want it to. All of this makes it easy to understand why many labs avoid automating or own expensive instrumentation that goes unused.
The Field Support Scientists (FSS) at Promega can provide the support you need to get started with automation or optimize your current HT workflow. Our team of automation experts can offer assistance with most of the leading laboratory automation providers in the world and help you develop and implement an automated nucleic acid purification solution on a variety of sample types and instrumentation platforms customized to the needs of your laboratory.
More information and resources for exploring Lab Automation can be found at the Lab Automation Resource Center.
Custom HT Nucleic Acid Purification
Implementing automated nucleic acid purification technologies onto your high-throughput workflow can be challenging and time-consuming. Our Field Support Scientists can provide the support you need to get started.
Promega Products for RNA Purification
Choosing the right RNA purification method and products is vital for obtaining high-quality RNA that meets the demands of sensitive downstream applications.
The Promega portfolio of RNA purification products provides reliable solutions designed to optimize workflow efficiency and data quality. For a complete list of RNA purification kits, please visit our RNA Extraction page.
Featured Systems:
ReliaPrep™ RNA Miniprep System
SV Total RNA Isolation System
The SV Total RNA Isolation System (Cat.# Z3101) is a versatile system that can process a wide range of sample sizes and types, delivering high yields of pure RNA suitable for downstream applications.
Protocol
TM048 SV Total RNA Isolation System Technical Manual
ReliaPrep™ miRNA Cell and Tissue Miniprep System
The ReliaPrep™ miRNA Cell and Tissue Miniprep System (Cat.# Z6210) is primarily used for small RNA (including miRNA), however this system can also be used to purify total RNA, including genomic RNA, from cells and tissues with modifications to the protocol.
Protocol
TM469 ReliaPrep™ miRNA Cell and Tissue Miniprep System Technical Manual
ReliaPrep™ FFPE Total RNA Miniprep System
The ReliaPrep™ FFPE Total RNA Miniprep System (Cat.# Z1001) uses optimized lysis condition to reverse the cross linking and other induced modifications that can result from the formalin fixation process. The protocol is completed in 1.5 hours and uses a deparaffinization method that does not rely on xylene or other organic solvents. By eliminating the washing steps involved in xylene deparaffinization, this method can help retain small RNA fragments.
Protocol
TM353 ReliaPrep™ FFPE Total RNA Miniprep System Technical Manual
Maxwell® RSC simplyRNA Kits for Blood, Cells and Tissue
Maxwell® RSC simplyRNA Kits for Blood (Cat.# AS1380), Cells (Cat.# AS1390) and Tissue (Cat.# AS1340) offer efficient RNA extraction from blood, cells or tissue samples. Designed for use with Maxwell® RSC Instruments, which simplifies the process by using preprogrammed methods and predispensed reagent cartridges. The kits provide high-quality RNA suitable for RT-qPCR, sequencing and other downstream applications.
Protocol
TM416 Maxwell® RSC simplyRNA Cells and SimplyRNA Tissue Kits Technical Manual
TM417 Maxwell® RSC simplyRNA Blood Kit Technical Manual
Maxwell® RSC miRNA from Tissue Plasma and Serum Kits
The Maxwell® RSC miRNA Tissue Kit (Cat.# AS1460) purifies total RNA, including microRNA (miRNA), from 1–48 tissue samples in about 90 minutes. The Maxwell® RSC miRNA Plasma and Serum Kit (Cat.# AS1680) will purify total RNA, including miRNA, from 1–48 samples in about 70 minutes. The kits work with both the Maxwell® RSC and RSC 48 instruments. The Maxwell® RSC Instrument can process from 1 to 16 samples and the Maxwell® RSC 48 can process from 1 to 48 samples in a single run. The Maxwell® RSC miRNA Tissue and miRNA Plasma and Serum Kits are also compatible for use on the Maxwell® CSC Instrument in RUO Mode.
Protocol
TM441 Maxwell® RSC miRNA Tissue Kit Technical Manual
TM546 Maxwell® RSC miRNA Plasma and Serum Kit Technical Manual
Maxwell® RSC RNA FFPE Kit
The Maxwell® RSC RNA FFPE Kit (Cat.# AS1440) offers automated extraction of RNA from FFPE tissue samples without using hazardous organic solvents. Used with the Maxwell® RSC Instruments, the simple protocol is easy to follow, and the final extraction is automated using a preprogrammed method. The kit provides RNA suitable for a variety of downstream applications.
Protocol
TM436 Maxwell® RSC RNA FFPE Kit Technical Manual
Maxwell® RSC Plant RNA Kit
Maxwell® RSC Plant RNA Kit (Cat.# AS1500) offers automated extraction of RNA from a variety of plant tissues using the Maxwell® RSC Instrument and prefilled reagent cartridges. It provides efficient and consistent RNA isolation, suitable for applications such as RT-qPCR and sequencing.
Protocol
TM459 Maxwell® RSC Plant RNA Kit Technical Manual
Maxwell® HT SimplyRNA
Maxwell® HT simplyRNA Kit, Custom supports high-throughput extraction of RNA from many sample types.
The Maxwell® HT miRNA Plasma/Serum Kit streamlines extraction of high-quality, amplifiable miRNA from plasma, serum or enriched exosomes. This kit is designed for use with laboratory automation and is supported by the Promega Scientific Applications team and Field support scientists who can assist laboratories in implementing the reagents.
References
- Chomczynski, P and Sacchi, N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Ananl. Biochem. 162, 156–9.