Understanding Cellular Biology in Real Time
1Cellular Reporters Senior Product Manager, 2Cell Health and Functional Analysis Product Manager, Promega Corporation
Publication Date: February 2019; tpub_207
Abstract
Live cell kinetic assays for measuring cell health and reporter activity are excellent tools to study the long-term effects of drug compounds on cultured cells, while providing a streamlined approach to data collection. These assays are non-lytic, making it possible to read and re-read the same sample at various time points throughout the experiment for up to three days. Collecting multiple time point readings from one sample allows the study of dynamic biology in real time, and removes the guesswork around time point collection. Additionally, multiple time point readings from one sample decreases assay variability because any change in treatment response can be studied in the same cell population.
Here we present examples of biological questions that can be addressed using Promega real-time assays on the GloMax® Discover instrument. We also include key considerations that can improve your success.
Repeated measurement of the same cell population over time with a real-time assay provides researchers the ability to generate more useful data from a single assay. You can go beyond simply determining if cells were affected by a treatment to understanding when and how quickly a treatment affected cells. This ability to kinetically measure cell viability, cytotoxicity or apoptosis in a single population of cells over time offers enhanced analysis of treatments, while reducing the resources needed, and variability caused by assaying multiple cell populations.
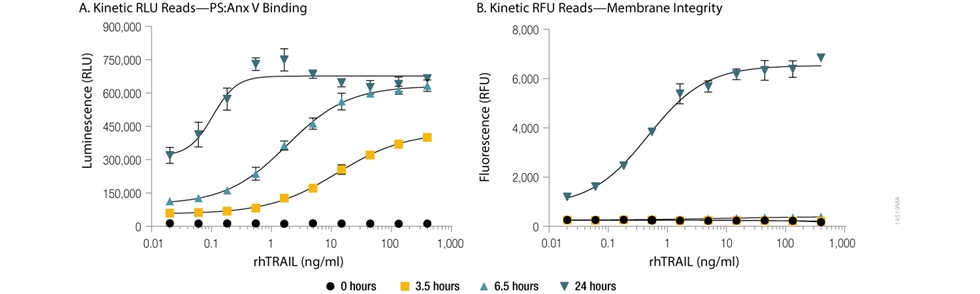
With the RealTime-Glo™ Annexin V Apoptosis and Necrosis assay, both Annexin V binding to phosphatidylserine (PS), a classic measure of the apoptotic phenotype, and necrosis as measured by membrane permeability can be monitored in the same well repeatedly over time. The binding of labeled Annexin V proteins to PS generates a luminescent signal, while the necrosis detection reagent generates a fluorescent signal. How these signals change over time after treatment can be used to determine if the treatment induced apoptosis or another form of cell death (Figure 1).
Figure 1. Annexin V binding to phosphatidylserine (PS:Anx) and loss of membrane integrity in real-time. U937 cells were incubated at 37°C/5% CO2 in the presence of serial dilutions of rhTRAIL and the RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay Reagents. Background subtracted luminescence, RLU (Annexin V binding to phosphatidylserine, Panel A) and background subtracted fluorescence, RFU (membrane integrity, Panel B) were measured at 0, 3.5, 6.5, and 24 hours on the GloMax® Discover instrument. The time-dependent increase in luminescence (due to Annexin V binding to phosphatidylserine, Panel A) that occurs prior to the time-dependent increase in fluorescence (due to loss of membrane integrity, Panel B) reflects apoptosis followed by secondary necrosis.
Assays that monitor cell health in real-time, like the RealTime-Glo™ Annexin V Assay, save cells, plates, and your time. Setting up one plate that can be read repeatedly over the time course of the experiment reduces the need to set up many parallel plates to be read with endpoint assays and then discarded. As Figure 2 shows, one plate monitored with the RealTime-Glo™ Annexin V Assay can replace seven plates using an endpoint assay to determine caspase activation. Figure 2 demonstrates that using real-time assays to monitor cell health generates more data per well, helping researchers better understand the biology of their assay systems.

Figure 2. Left Panel: Real-time assay. Data for each dose was obtained at multiple time points using a single assay plate with one RealTime-Glo™ Annexin V Apoptosis Assay reagent addition. Right Panel: Endpoint assay. Seven separate plates were used to collect parallel data using an endpoint assay.
Monitoring Bioluminescent Reporter Activity in Real Time
Although luciferase reporter assays are often performed using lytic end-point methods, real time monitoring of reporter activity provides an option to better understand the kinetics of a wide variety of cellular responses. NanoLuc® luciferase provides a small, sensitive bioluminescent reporter that is compatible with real-time monitoring from several hours to days when detected using the Nano-Glo® Live Cell Substrates. The Nano-Glo® Live Cell Assay System provides the brightest signal and widest dynamic range for <2 hour experiments. The Nano-Glo® Endurazine™ Live Cell Substrate provides the lowest initial signal intensity but has maximal stability for multi-day experiments. The Nano-Glo® Vivazine® Live Cell Substrate provides an intermediate option of brightness and stability (Figure 3). Together these substrates provide options that balance brightness and signal stability to accommodate a variety of experimental goals.
Figure 3. Choose the best combination of signal intensity and stability by comparing Nano-Glo® Endurazine™ and Nano-Glo® Vivazine® Substrates with Nano-Glo® Live Cell Assay System. A plasmid encoding NanoLuc® luciferase expressed from the PGK promoter was transiently transfected into HEK293 cells and luminescence signal intensity was measured as indicated.
The destabilized NanoLuc® reporter NlucP (NanoLuc®-PEST) is a short half-life reporter that turns over quickly in the cell. When used in a live cell format it provides a way to measure dynamic changes in a transcriptional response following compound treatment. Figure 4 demonstrates how a cAMP response element (CRE)-NlucP reporter and the Nano-Glo® Vivazine® Substrate can be used to measure the dose-response time course of transcriptional activation that occurs following forskolin-mediated increase in intracellular cAMP. Both the kinetics of transcriptional activation as well as the subsequent decline can be observed in a dose-dependent manner over the 10-hour study.

Figure 4. HEK293 cells were transiently transfected with a construct expressing NlucP from a promoter with cAMP response elements (CRE). Vivazine® substrate was added to cells at time zero, and luminescence was measured continuously using a GloMax® Discover instrument at 37°C. After approximately 2 hours, varying concentrations of forskolin were added to induce expression of NlucP by increasing the intracellular cAMP concentration. The graph shows a ten-hour time course with per-well normalization of the data. The substrate detects a dose-dependent increase of CRE reporter activity in response to increasing cAMP levels.
The Nano-Glo® Live Cell Substrates can also be used with NanoBiT® reporter technology to assay the kinetics of protein interactions or quantify real-time changes in protein abundance. Figure 5 demonstrates the use of the Nano-Glo® Endurazine™ and Vivazine® Substrates to monitor real-time expression levels of HiBiT-tagged proteins in cells expressing LgBiT, where HiBiT binds to LgBiT to form the active NanoBiT® luciferase. In Figure 5, the HiBiT tag was introduced via CRISPR-Cas9 gene editing of the N-terminal BRD4 locus to monitor endogenous expression conditions. Protein levels were quantified following treatment with ARV771, a compound that targets BRD4 for degradation. Quantification of the HiBiT tag in live cells allows for real-time tracking of BRD4 protein levels and demonstrates rapid depletion of the protein following ARV-771 treatment, and that the depletion persists for at least 24 hours.
Figure 5. Kinetically monitor endogenous BRD4 levels. HEK293 cells stably expressing LgBiT were modified via CRISPR-Cas9 to create cells expressing a HiBiT-BRD4 fusion at the endogenous BRD4 locus. Expression levels of a HiBiT-BRD4 fusion were monitored in real time using Endurazine™ and Vivazine® substrates following treatment with ARV-771, a BRD4-targeted protein degrader (PROTAC).
Key Considerations for Live-Cell Assays
While these results are exciting, several challenges remain in implementing real-time live-cell assays. Many researchers store their experimental culture plates in a gas, humidity and temperature-controlled incubator and then manually transfer the plates to a plate reader when it is time to collect a measurement. This is the most economical approach but it has its challenges. This manual transfer approach allows you to easily remove the plate lid before reading to get the best possible signal from your plate reader, but the longer the plate is outside of the controlled incubator conditions, the greater the variability of results can occur. Some researchers may also be concerned about the potential for microbial contamination when the plate lid is removed.
To overcome these concerns, one approach is to use a plate reader capable of controlling the environmental conditions. In this way you can simply insert your plate into the reader and collect measurements while maintaining the desired gas and temperature throughout your experiment. While this approach allows for easy time point measurements without the need for manual plate transfers, the downside is that plate readers do not control humidity, which causes two issues. First, evaporation in outer wells may still occur, limiting assays to use only the inner 60 wells of a 96-well plate. This limits throughput as plate layouts need to be adapted for the limited number of wells. Second, the lack of humidity control and the need to keep the plate lid on also means all sample measurements will be made through the lid. Taking assay readings through the lid reduces assay sensitivity, along with increasing well-to-well crosstalk. Condensation may also collect on the lid throughout the experiment, negatively impacting assay readings.
Some alternative considerations to make both methods easier include the use of optically-clear gas permeable plate seals. These seals eliminate the need to use the traditional plate lid with hopes of reducing well-to-well crosstalk since you are not reading through the plate lid, but are keeping each well protected from the environment with the seal (Figure 6). An alternative is the use of CO2-independent media that is tolerant to pH changes and keeps cells viable over the course of the experiment. The use of CO2-independent media allows you to either manually move plates to and from the incubator without the worry of assay variation due to short periods of uncontrolled environment, or to use a plate reader without dedicated environmental controls (Figure 7).
Figure 6. Crosstalk for various lids and seals. The plastic lids that come with Coring plates Cat.# 3917 have significantly higher crosstalk compared to the Breathe-Easy® Film (USA Scientific, Cat.# 9123-6100), or no lid. The Breathe-Easy® Film has higher crosstalk compared to no lid.
Figure 7. Signal/background counts by cell number of serially-diluted K562 cells in CO2 independent media for 24 hours at 37°C in a GloMax® Discover. Five-thousand cells/well were diluted 2-fold down to 20 cells/well. Similar signal/background levels and sensitivity was observed for both the Breathe-Easy Film (USA Scientific, Cat. # 9123-6100) and the 96-well plastic lid.
The GloMax® Discover is integrated with Promega assays to streamline the user experience while maximizing assay performance. Over 50 preset protocols, including long-term kinetic protocols for real-time assays, allow the user to get up and running faster (Figure 8).
Figure 8. Heat map graphical display from the GloMax® Discover System with kinetic plots of each well. Blue wells represent samples with lower signal. Red wells represent samples with higher signal.
Conclusion
Real-time live cell analysis using only a plate-reading luminometer offers researchers new avenues to simply and economically explore dynamically changing biology. The above examples illustrate the power that real-time assays and compatible instruments give to researchers.
Learn more about our portfolio of live-cell kinetic assays!
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Landreman, A., Bach, M., Bjerke, M. Understanding Biology in Real Time. [Internet] February 2019; tpub_207. [cited: year, month, date]. Available from: https://www.promega.com/resources/pubhub/2019/understanding-cellular-biology-in-real-time/
American Medical Association, Manual of Style, 10th edition, 2007
Landreman, A., Bach, M., Bjerke, M. Understanding Biology in Real Time. Promega Corporation Web site. https://www.promega.com/resources/pubhub/2019/understanding-cellular-biology-in-real-time/ Updated February 2019; tpub_207. Accessed Month Day, Year.
Nano-Glo substrates:
(a)BY USE OF THIS PRODUCT, RESEARCHER AGREES TO BE BOUND BY THE TERMS OF THIS LIMITED USE LABEL LICENSE. If researcher is not willing to accept the terms of this label license, and the product is unused, Promega will accept return of the unused product and provide researcher with a full refund. Researcher may use this product for research use only; no commercial use is allowed. Commercial use means any and all uses of this product by a party in exchange for consideration, including, but not limited to (1) use in further product manufacture; (2) use in provision of services, information or data; and (3) resale of the product, whether or not such product is resold for use in research. Researcher shall have no right to modify or otherwise create variations of the product. No other use or transfer of this product is authorized without the prior express written consent of Promega. For uses of Nano-Glo® -branded reagents intended for energy transfer (such as bioluminescence resonance energy transfer) to acceptors other than a genetically encoded autofluorescent protein, researchers must: (a) use NanoBRET®-branded energy acceptors (e.g., BRET-optimized HaloTag® ligands) for all determinations of energy transfer activity by this product; or (b) contact Promega to obtain a license for use of the product for energy transfer assays to energy acceptors not manufactured by Promega. With respect to any uses outside this label license, including any diagnostic, therapeutic, prophylactic or commercial uses, please contact Promega for supply and licensing information. PROMEGA MAKES NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING FOR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH REGARD TO THE PRODUCT. The terms of this label license shall be governed under the laws of the State of Wisconsin, USA.
(b)Patent Pending.