High-Throughput Cytokine Detection with Lumit® Technology
Kendra Hanslik, Promega Corporation
Introduction
In the rapidly evolving landscape of biomedical research and drug discovery, the ability to efficiently screen and quantify biomolecules like cytokines is paramount. Cytokines, small proteins crucial in cell signaling, play pivotal roles in the immune response and are involved in a variety of diseases, including autoimmune disorders, infections, and cancers. Traditional methods for cytokine detection often involve complex, time-consuming protocols that can become bottlenecks in high-throughput screening (HTS) settings. On the contrary, Lumit® technology enables the ability to streamline and accelerate cytokine detection, especially for high-throughput application.
Conventional Methods for Cytokine Detection
Enzyme-linked immunosorbent assay (ELISA)
ELISA is one of the most widely used methods for detecting and quantifying soluble cytokines. This technique is based on the principle of antibodies binding to specific antigens. ELISA can be configured to different formats, with the sandwich ELISA being the most common for cytokine detection. Despite its widespread use, ELISA is time-consuming, typically requiring multiple wash steps and several hours to complete. Due to limited assay sensitivity, ELISA is typically performed in a 96-well format, limiting high throughput approaches.
Flow cytometry
Flow cytometry is another powerful technique that allows for multiparametric cell analysis, enabling cell size and granularity to be assessed simultaneously. When combined with fluorescently labeled cytokine-specific antibodies, flow cytometry can detect and quantify cytokine production at a single-cell level. However, flow cytometry requires expensive equipment and considerable expertise to perform and analyze the results. While it is possible to run multiple samples at once, specialized techniques or equipment are needed to achieve this. Cells must also remain in suspension for analysis, which may not be ideal for certain sample types or assays.
Western blots
Western blotting is an additional method for detecting specific proteins. While not as sensitive as ELISA, western blotting is useful for confirming the presence of cytokines and assessing their molecular weight in various samples including tissues and cells. However, it is labor-intensive, requiring multiple days to obtain data that is mainly qualitative, though densitometry can provide a semi-quantitative analysis. Its multi-step protocol and time-consuming nature make it unsuitable for high-throughput screening.
No-Wash Lumit® Immunoassays for Cytokine Detection
Lumit® Immunoassays represent a significant advancement in high-throughput cytokine detection. First, they are fully homogeneous, eliminating cumbersome steps of sample transfer and washing commonly found in traditional assays. This not only saves precious time but also reduces the potential for errors, improving assay reliability. Moreover, the adaptability of Lumit® assays for automation in a 384-well plate format is a significant advantage for laboratories aiming to scale up their screening capabilities. The compatibility of these assays with various liquid dispensing instruments further underscores their versatility in different research settings.
The Lumit® Immunoassay is based on NanoLuc® Binary Technology (NanoBiT), a structural complementation system containing two subunits, Small BiT (SmBiT) and Large BiT (LgBiT). These subunits have been optimized for stability and have a low affinity for each other, only forming a functional luciferase enzyme when both subunits come into proximity. For cytokine detection, primary antibodies specific to a target cytokine are chemically labeled with SmBiT and LgBiT. In the presence of the target cytokine, the SmBiT and LgBiT subunits come into proximity, reassembling into a functional luciferase enzyme that generates a luminescent signal proportional to the amount of cytokine present. The video below illustrates this binding concept and demonstrates the simple, no-wash workflow.

Lumit® Immunoassay Performance for High Throughput Applications
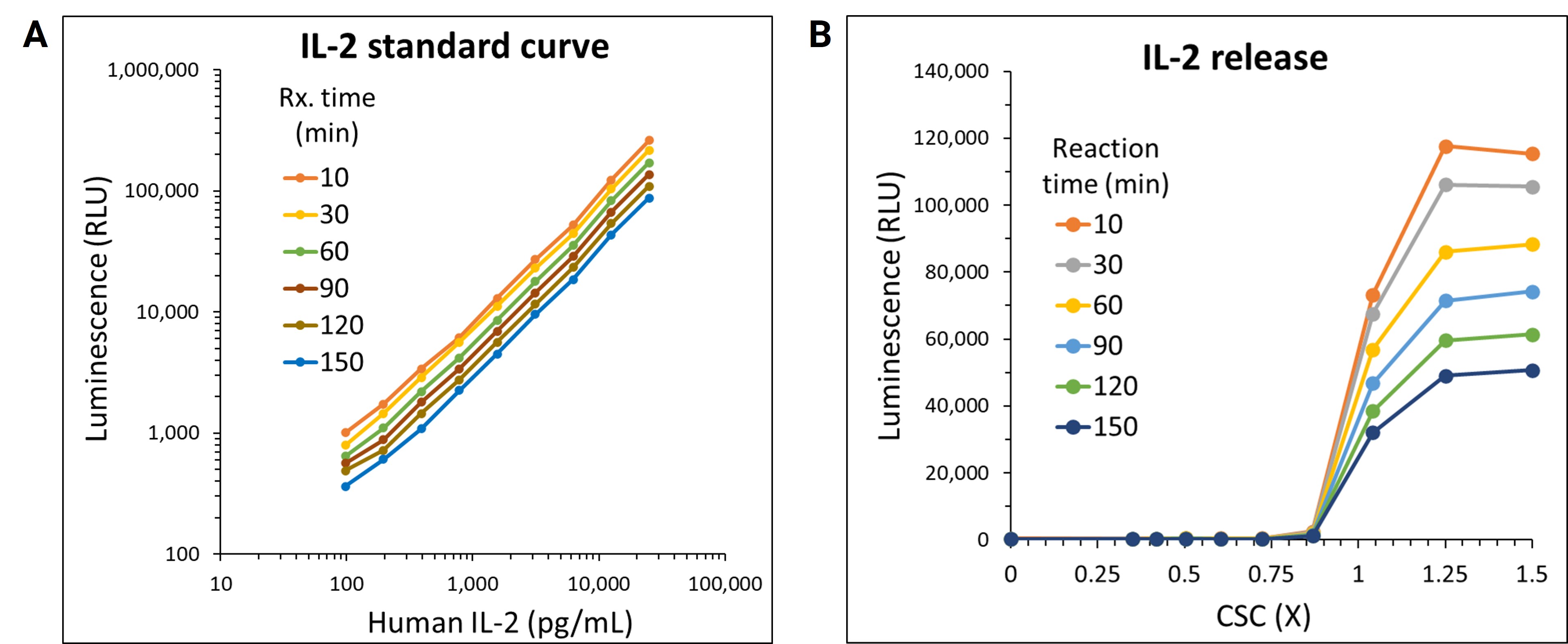
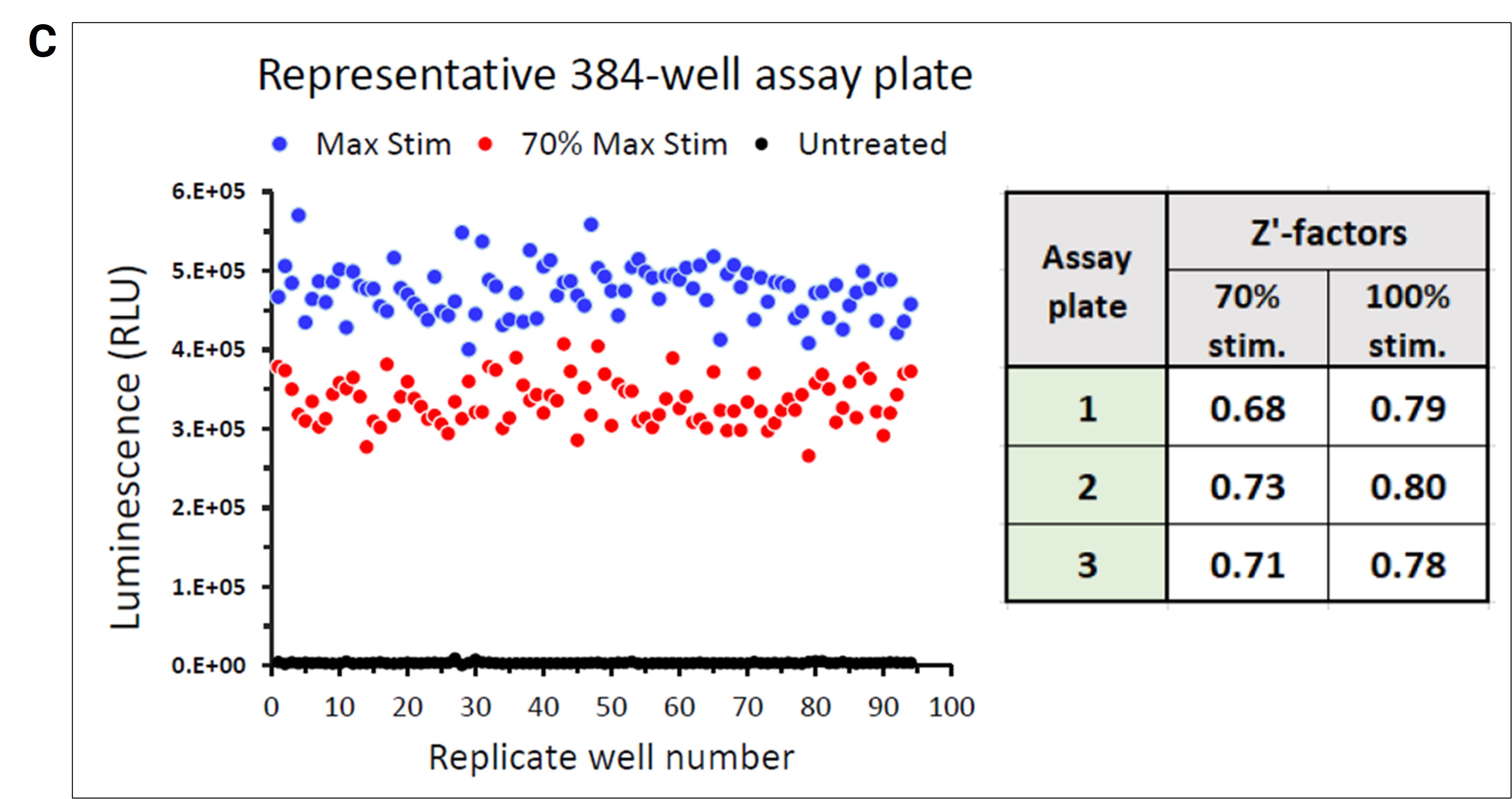
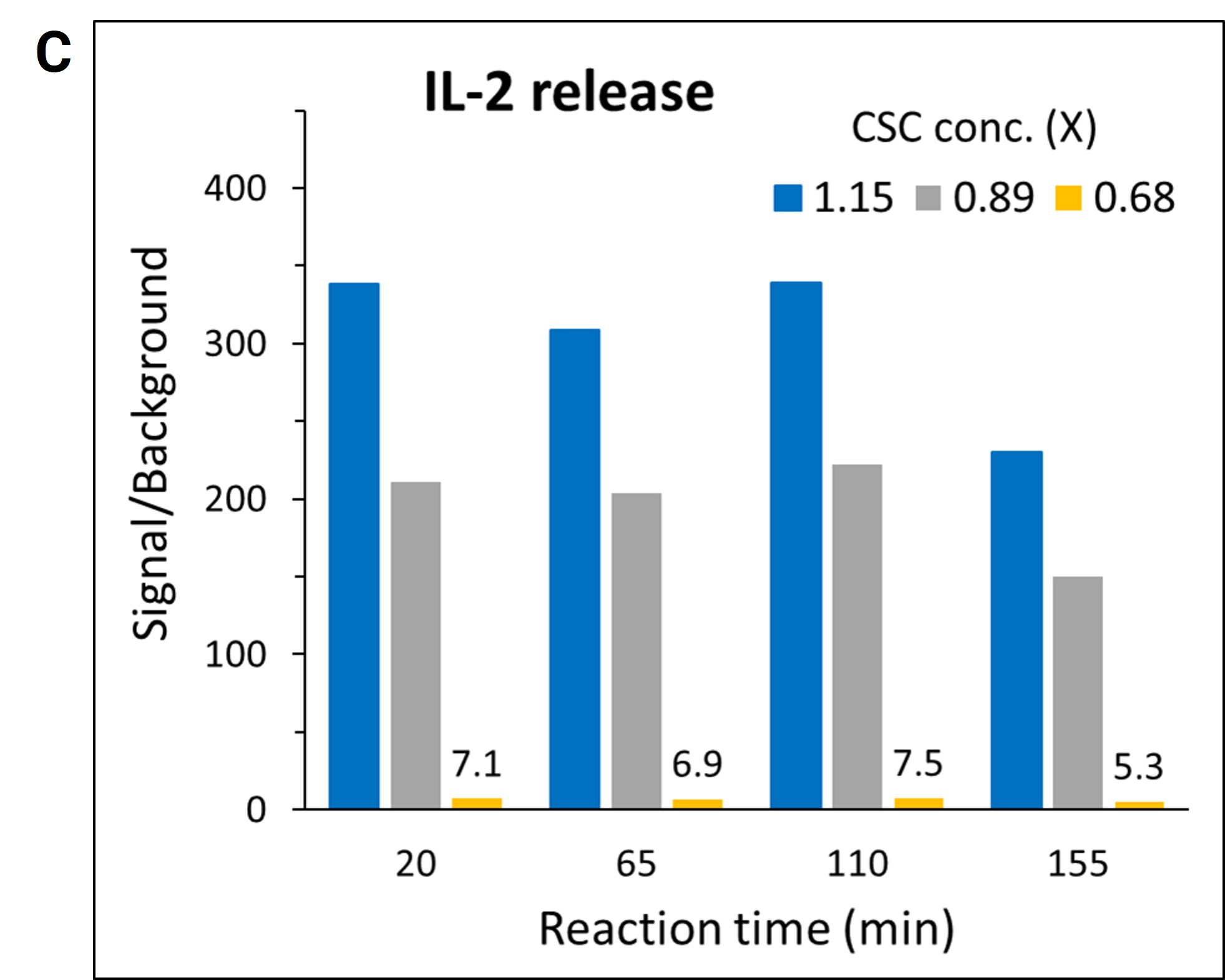
The practical application of Lumit® technology was demonstrated by a team of Promega scientists using a high throughput approach with human peripheral blood mononuclear cells (PBMCs) following a Cell Stimulation Cocktail (CSC) treatment. The use of automated liquid handling for setup, treatment and subsequent Lumit® Immunoassay to assess the release of IL-2 was performed in 384-well plates. A variety of liquid handling instruments were used including Formulatrix Mantis® for dispensing cells and Lumit® antibody mixtures, Tecan EVO with an MCA 96 tip head for making and dispensing CSC serial dilutions, and a Thermo Fisher Multidrop™ Combi_nL Reagent Dispenser for dispensing Lumit® Detection Reagent B and media backfill. The collected data showcases Lumit® Immunoassay compatibility with high-throughput applications. The assay exhibited excellent linearity and stable kinetics (half-life ~2 hours), facilitating the generation of reliable data in 384-well plates (Figures 1A & 1B). The quality and reliability of HTS data is further demonstrated by the Z’ determination results yielding excellent statistical performance (response CVs < 10%, Z’ values ≥ 0.68) generated using the Lumit® IL-2 Immunoassay (Figure 1C).
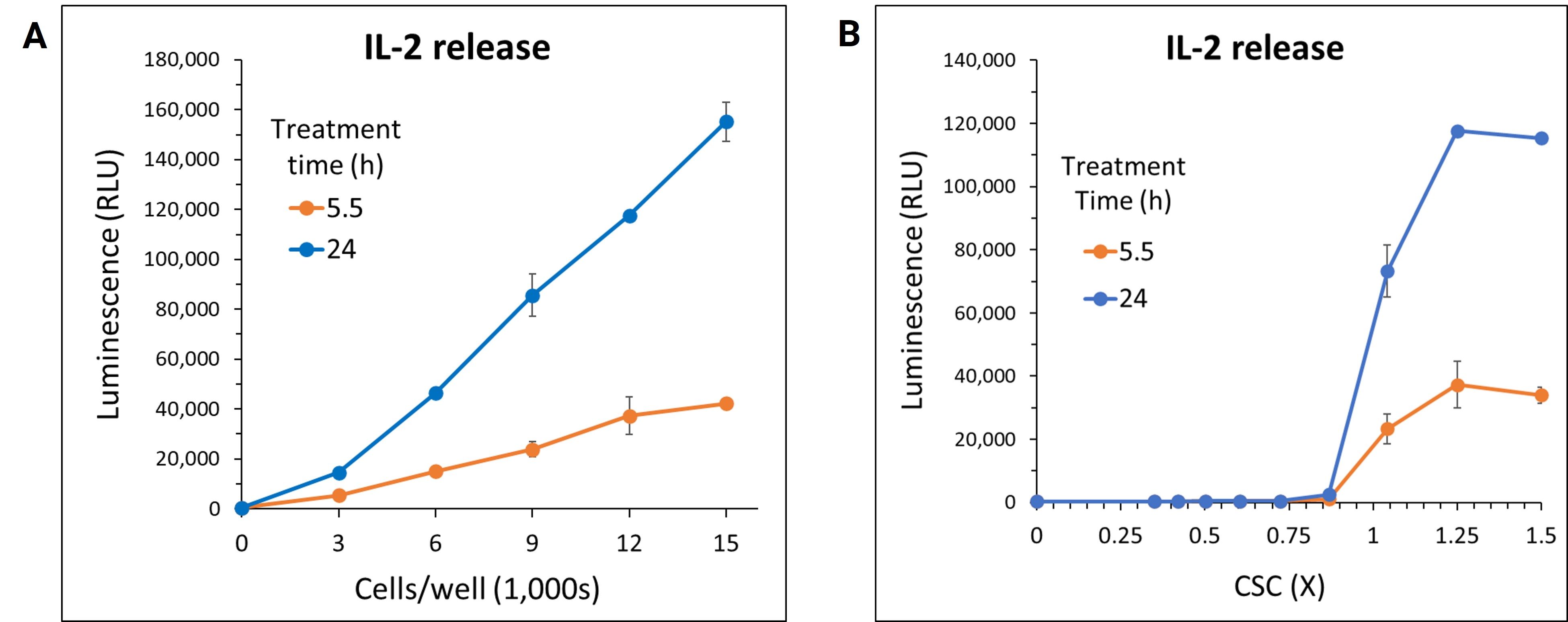
To achieve the best assay results, the number of PBMCs per well was optimized. Optimization of cell numbers revealed 12,000 cells/well was sufficient for offering a large assay window while providing signals well within the linear ranges of the cytokine standard curves (Figure 2A). In dose-response curves for IL-2 release following CSC treatment, signal-to-background (S/B) ratios exceeded 250-fold (Figures 2B & 2C). The assay’s high sensitivity and S/B ratio enable fewer cells and lower sample volumes to be used during HTS protocols. Furthermore, the sustained excellent S/B performance for at least 2 hours post-signal generation is particularly advantageous for batch plate processing, a common requirement in HTS workflows.
In conclusion, Lumit® Cytokine Immunoassays are a significant advancement in the field of cytokine detection. A simple workflow, compatibility with automation and 384-well plate format, and excellent assay performance characteristics positions Lumit® Immunoassays as a valuable tool for high-throughput screeners. By simplifying and improving the efficiency of cytokine measurement, Lumit® technology paves the way for faster, more effective drug discovery and biomedical research, ultimately contributing to the advancement of healthcare and treatment modalities.
For detailed insights into the performance of the Lumit® IL-2 Immunoassay or other cytokine immunoassays in high-throughput applications, download the poster: Lumit® Cytokine Assay Automation for High-Throughput Screening.
New to bioluminescent technology? Learn about the advantages of bioluminescent assays for HTS applications.
Learn how Lumit® Immunoassays compare to traditional immunoassays
Related Resources

Webinar: Lumit® Immunoassays: An Easier Faster Method for Analyte Detection
Part 1 of the Lumit™ Technology Webinar Series
Webinar: Using Lumit™ Technology to Address Inflammasome-mediated Cytokine Release
Part 2 of the Lumit™ Technology Webinar Series