High Protein Yield and Purity with the HaloTag® Protein Purification System
Promega Corporation
Publication Date: 2010
Abstract
The HaloTag® Protein Purification System is based on the novel HaloTag® protein tag, which enables efficient, specific capture of a HaloTag®-fused protein of interest through covalent immobilization. At the same time the HaloTag® technology enhances the expression of soluble recombinant proteins in E. coli. Purification using HaloTag® technology results in target proteins of high yield and purity, with no HaloTag® protein tag carryover, through the process of covalent fusion protein capture and subsequent target protein release by TEV protease cleavage of an optimized TEV recognition sequence embedded in the Flexi® Vector System.
Introduction
HaloTag® Technology is based on a 34kDa, monomeric protein tag modified from a bacteria dehalogenase, designed to covalently bind to synthetic HaloTag® ligands (1) (2) . A variety of HaloTag® ligands are available for in vivo and in vitro applications, such as fluorescent ligands for protein detection and cell imaging, and HaloLink™ Resin for isolating protein complexes as well as purification.
HaloTag® Technology has been optimized for compatibility with many protein expression systems including E. coli, mammalian cells and cell-free expressions systems. When studied with more than 20 difficult-to-express human proteins, the HaloTag® protein fusion tag has been shown to outperform GST, MBP and His•Tag® in producing soluble fusion proteins and purifying target proteins of higher yield and purity from E. coli (3) .
When studied with more than 20 difficult-to-express human proteins, the HaloTag® protein fusion tag has been shown to outperform GST, MBP and His•Tag in producing soluble fusion proteins and purifying target proteins of higher yield and purity from E. coli.
The HaloTag® Protein Purification System has three components: HaloLink™ Resin, TEV Protease and HisLink™ Resin. A streamlined protocol has been developed around these three components to complete the following four steps in purification (see also, Figure 1):
- Covalent binding for efficient and specific capture of a HaloTag® fusion protein onto HaloLink™ Resin. Covalent capture makes it possible to recover proteins expressed even at low levels.
- Wash away unbound protein contaminants; stringent washes are possible with minimal loss after the HaloTag® fusion protein is covalently attached to HaloLink™ Resin.
- Release the target protein from the HaloTag® protein tag by on-resin TEV Protease cleavage, leaving the HaloTag® protein tag itself permanently attached to the HaloLink™ Resin.
- The TEV Protease included in the system [with an N-terminal (HQ)3 tag] is removed from target of interest by HisLink™ Resin.
 Figure 1. Schematic diagram of protein purification using HaloTag® Technology.
Figure 1. Schematic diagram of protein purification using HaloTag® Technology. The result is purified protein of interest, typically recovered with high yield and purity and without HaloTag® protein tag carryover. One simple buffer, the HaloTag® purification buffer (HT buffer: 50mM HEPES (pH 7.5), 150mM NaCl with optional additives 1mM DTT, 0.5mM EDTA, and 0.005% IGEPAL® CA-630; HT buffer referred to in this article, contained all the optional additives), is compatible with all purification steps. If needed, the system can also be optimized to include stringent washing steps by altering buffer compositions to remove recalcitrant contaminants (see HaloTag® Protein Purification System Technical Manual #TM312 for details).
Using HaloTag® Technology in E. coli-Based Expression and Purification
HaloTag®-based protein purification can be applied to proteins expressed in multiple systems, such as E. coli, mammalian cells and cell-free systems. The HaloTag® Protein Purification System Technical Manual, TM312 covers conditions developed and optimized for HaloTag® fusions expressed in E. coli, with additional recommendations for mammalian cells. Table 1 contains a quick guide to basic steps and required materials for E. coli-based HaloTag® expression and purification.
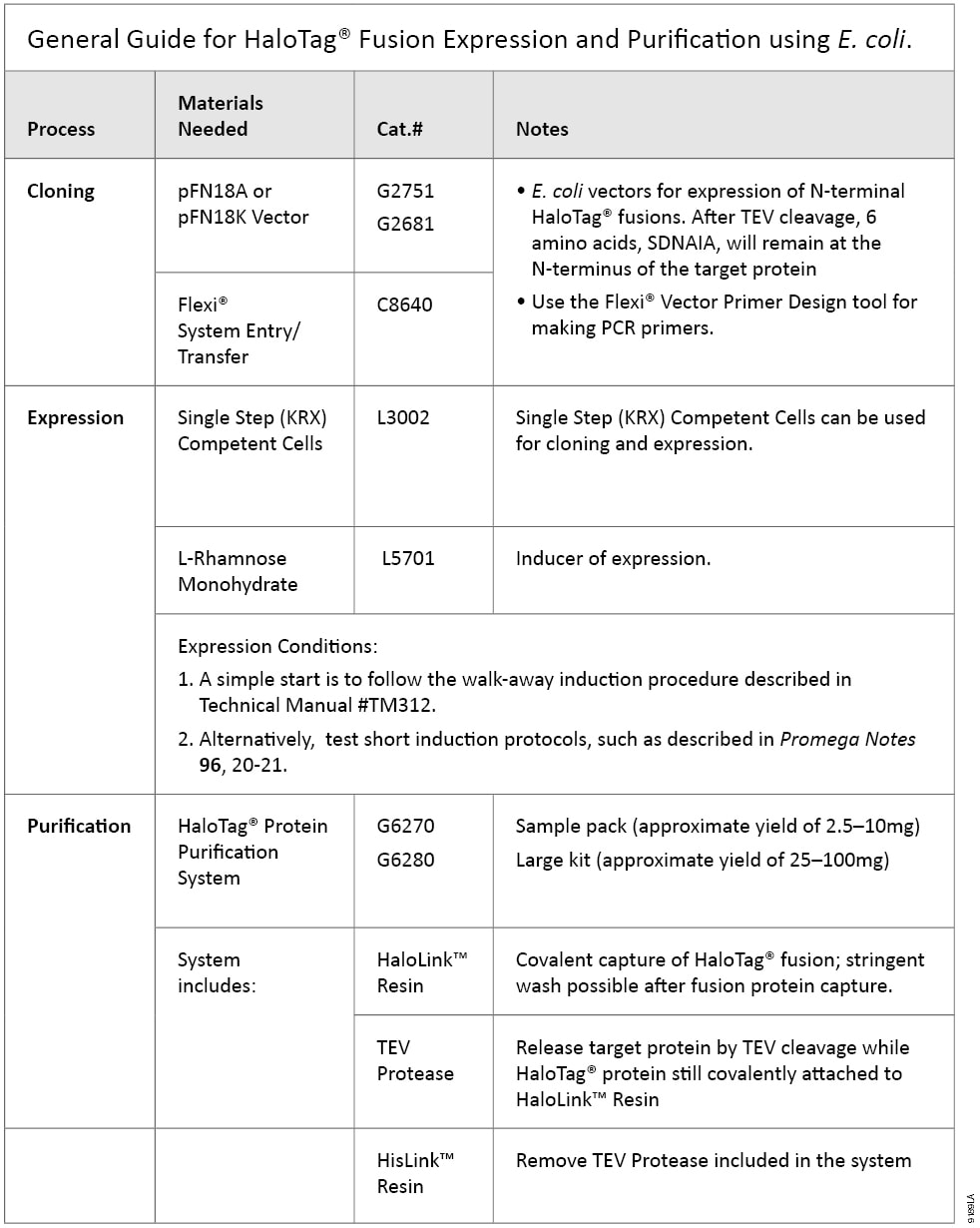 Table 1. General Guide for HaloTag® Fusion Expression and Purification using E. coli.
Table 1. General Guide for HaloTag® Fusion Expression and Purification using E. coli. Features of HaloTag® Protein Purification System
In equilibrium-based affinity purification, protein is constantly exchanged between two states, either bound or not bound to the affinity resin (e.g., GST to GSH resin and His•Tag® to Ni2+-charged solid supports). This can lead to issues such as reduced binding efficiency when the fusion protein expression is low and the loss of protein targets during extensive washing. Additionally, large fusion tags, such as GST and MBP, often need to be removed from the protein of interest, usually by specific protease cleavage followed by affinity removal of the free tags. This latter step can be problematic, as the tags themselves are hard to eliminate and thus become a contaminant in the final purified protein.
HaloTag® technology-based purification, because of its covalent protein capture, provides solutions to these common problems: (3) .
- Covalent protein capture renders the immobilization process irreversible, so that the binding of fusion proteins is less affected by expression level.
- Extensive and stringent washes are possible with minimal loss of target protein, because;
- HaloTag® itself will not elute with target of interest, as it is permanently bound and remains on the HaloLink™ Resin after on-column TEV Protease cleavage. Upon TEV Protease removal, the target protein, typically of high yield and purity, is recovered.
The benefits of HaloTag® technology-based expression and purification in E. coli are demonstrated in the following three examples. All purifications were performed using a batch/column combination method as described in TM312. Specifically, cells from 50ml of expression culture were collected, resuspended in 5ml of HT buffer, disrupted by sonication and centrifuged at 10,000 × g, 4°C for 30 minutes to collect soluble supernatant; a ratio of 5ml of expression cell lysate supernatant to 1ml of settled HaloLink™ Resin was used. The amount of TEV Protease used was about 300units/ml of settled HaloLink™ Resin, and 50µl of 50% HisLink™ Resin was used to remove TEV Protease. The ratios are listed in Table 2.
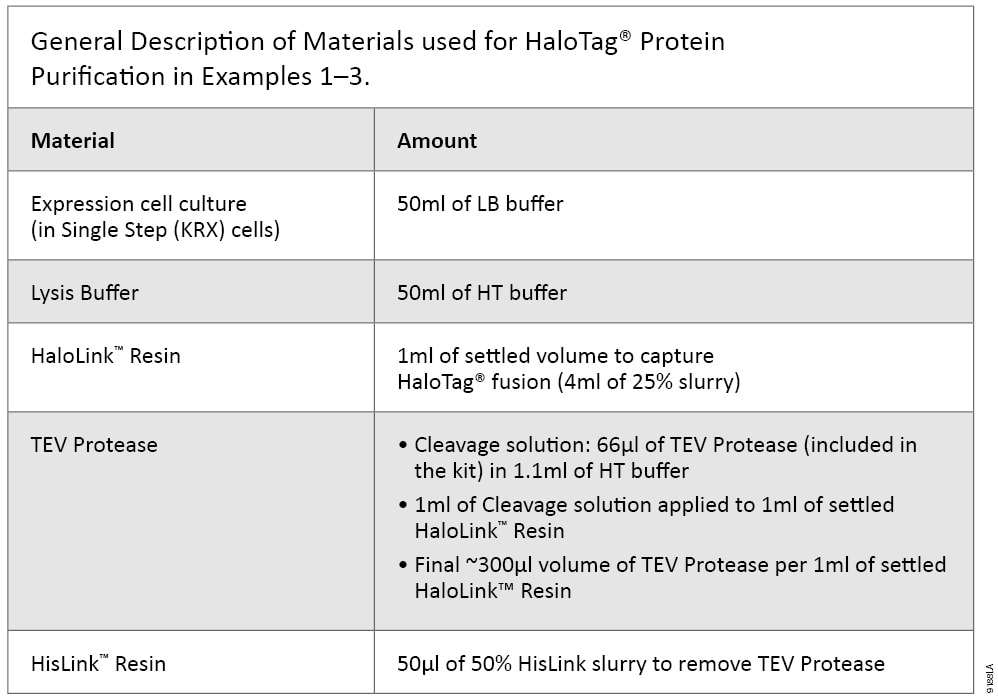 Table 2. General Description of Materials used for HaloTag® protein purification in examples 1–3.
Table 2. General Description of Materials used for HaloTag® protein purification in examples 1–3.
We compared samples from different purification steps using SDS-PAGE and/or an activity assay when applicable. The samples analyzed are described below with indicated nomenclature:
- S: Soluble cell lysate supernatant after cell lysis and high-speed centrifugation; this sample contains soluble fusion protein.
- S-Tev: Soluble cell lysate supernatant treated by TEV Protease; this sample has HaloTag® protein as well as untagged target protein, which is the reference for total recoverable target.
- FT: Flowthrough; this sample has unbound fusion protein after capture by HaloLink™ Resin, indicating binding efficiency when compared to the fusion protein in sample S.
- E1: Recovered target protein after on-resin Tev cleavage; this sample has untagged target as well as TEV Protease.
- E2: Final purified protein after removing TEV Protease.
The proteins are concentrated ~2.5-fold in E1 and E2 as compared to other fractions, since 2ml of the elutions is collected compared to 5ml of the starting cell lysate. This concentrating factor is applied in normalizing data analysis.
Example 1: High Protein Recovery
This example demonstrates high protein recovery, by analyzing the final protein yield of firefly luciferase (ffLuc) compared to that in the starting crude cell lysate. Protein recovery was analyzed by comparing the band intensities of ffLuc in E2 to that in S-Tev on a Coomassie®-stained SDS-PAGE analysis as well as by assaying ffLuc activity in the samples.
Firefly luciferase (ffLuc) was purified from 50ml of culture using 1ml of settled HaloLink™ Resin by batch/column combination method as described in TM312 and in Table 2. Purified protein was of high purity (>90%) as judged by SDS-PAGE analysis, and of high recovery as determined by comparing the Coomassie®-stained ffLuc band intensity in E2 to that in S-Tev. Active protein recovery was greater than 75% of the starting material (E2 compared to S-TEV), suggesting efficient capture of fusion protein and protein release by TEV Protease cleavage.
 Figure 2. Quantitative recovery of firefly luciferase.
Figure 2. Quantitative recovery of firefly luciferase.
Cells from 50ml of LB culture expressing HaloTag®-ffLuc were collected, resuspended in 5ml of HT buffer, disrupted by sonication and centrifuged at 10,000 × g, 4°C for 30 minutes to collect soluble supernatant; 5ml of expression cell lysate supernatant was applied to 1ml of settled HaloLink™ Resin. After 1 hour binding at room temperature with end-to-end rotational mixing, HaloLink™ Resin was loaded onto a column (Pierce Cat.# 89897), washed with 20ml (20cv) of HT buffer. One milliliter (1ml) of cleavage solution was loaded on to the column (about 300units TEV/ml of settled HaloLink™ Resin). TEV Protease cleavage was performed for 1 hour at room temperature. Target protein was eluted with HT buffer, about 2ml of elution was collected. Finally, 50µl of 50% HisLink™ Resin was added to remove TEV Protease and incubated for 20 minutes at room temperature with end-to-end rotational mixing. For SDS-PAGE (Invitrogen Tris-Glycine 4-20%), lanes S, S-TEV and FT are equivalent to 10µl of original cell culture and E1 and E2 are equivalent to 25µl of original cell culture. For activity analysis, all samples (S, S-TEV, FT, E1 and E2) were diluted 1:100 with 1X Glo Lysis Buffer (Cat.# E2661), 50µl of the dilutions were mixed with 50µl of Luciferase Assay Reagent (Cat.# E1501) at room temperature for 5 minutes and read on a GloMax® Luminometer. Data for E1 and E2 were normalized to S and S-Tev samples considering a concentrating factor of 2.5.
Example 2: Recovery of Proteins From Low Expression-Mimicking, Diluted Lysates
To demonstrate the ability of HaloTag® technology to purify proteins of low abundance, we compared purification from a tenfold diluted lysate to that from undiluted expression cell lysate. For the dilution analysis, we used nonexpressing cell lysate, prepared from Single Step (KRX) Competent Cells without a construct, where 200ml of KRX cell lysate was made from 2L of culture. The diluted sample represents a low-expressing fusion of 10% of the original expression lysate.
All purifications essentially are equivalent in terms of total amount of fusion protein applied to 1ml of settled HaloLink™ Resin except for the concentration of fusion protein in the lysate. Three targets were tested: ffLuc, hRL (humanized Renilla luciferase) and cPKA (human catalytic subunit of protein kinase A) . In all cases, similar protein yields were obtained, when the same amount of fusion protein was applied to a set amount of HaloLink™ Resin. Table 3 shows starting materials and final protein yields. A representative purification comparison by SDS-PAGE is shown in Figure 3.
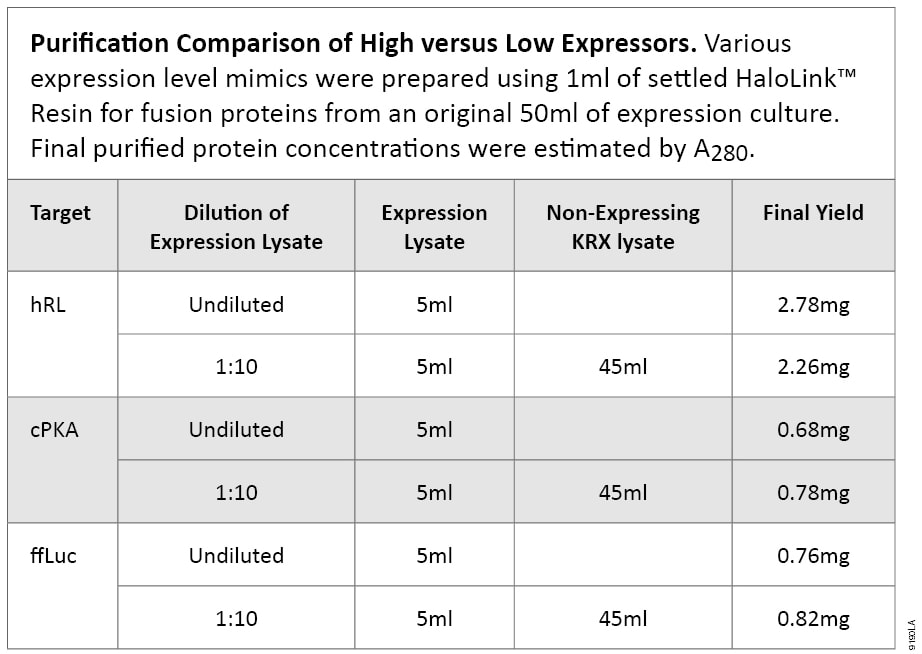 Table 3. Purification Comparison of High Versus Low Expressors.
Table 3. Purification Comparison of High Versus Low Expressors.
 Figure 3. Protein recovery from diluted lysates.
Figure 3. Protein recovery from diluted lysates.
Samples were prepared as described in Table 2. Cells from 100ml of LB culture expressing HaloTag® fusions and 2L of Single Step (KRX) Competent Cells without an expression construct were collected, resuspended in 1/10th culture volume using HT buffer and disrupted by sonication. Total lysates were centrifuged at 10,000 × g at 4°C for 30 minutes to collect soluble supernatant. Two parallel purifications were performed: 5ml of undiluted expression lysate supernatant or 50ml of diluted supernatant (5ml of undiluted expression lysate supernatant +45ml of KRX lysate) were applied to 1ml of settled HaloLink™ Resin. After 1 hour of binding at room temperature with end-to-end rotational mixing, HaloTag® Resin was loaded onto a column (Pierce Cat.# 89897), and washed with 20ml (20cv) of HT buffer. One milliliter (1ml) of cleavage solution was loaded onto each column (about 300 units of TEV/ml of settled HaloLink™ Resin). TEV cleavage was carried out for 1 hour at room temperature; target protein was eluted with HT buffer, and about 2ml of elution was collected. Finally, 50µl of 50% HisLink™ Resin was added to remove the TEV Protease and incubated for 20 minutes at room temperature with end-to-end rotational mixing. For SDS-PAGE analysis (Invitrogen Tris-Glycine 4–20%): Lanes S, E1 and E2 are for undiluted expression lysate; lanes S-1:10, E1-1:10 and E2-1:10 are for 10x diluted expression cell lysate; S is equivalent to 1µl of undiluted expression cell lysate, S-1:10 is equivalent to 0.1µl of undiluted expression cell lysate +0.9µl of KRX lysate; E1, E2, E1-1:10 and E2-1:10 are equivalent to 2.5µl of original undiluted expression cell lysate.
Example 3: Removal of E. coli Chaperonins: An Example of GroEL
E. coli chaperonins (e.g., GroEL and DnaK) can assist protein folding (4) . They are often copurified with recombinant proteins from E. coli and need to be removed by affinity chromatography. In this example, we successfully removed GroEL from human cPKA by two methods:
- The lysate was pretreated with a mixture of ATP/Mg2+ to dissociated chaperonin/protein complex prior to binding to HaloLink™ Resin, where HaloTag® protein capture was not affected by the treatment; and
- Chaperonin was removed on-column with ATP/Mg2+ after fusion protein binding to HaloLink™ Resin, where covalent capture ensured minimal protein loss during this treatment. The final proteins recovered were similar in both cases (Figure 4), demonstrating the versatility of the HaloTag® Protein Purification System.
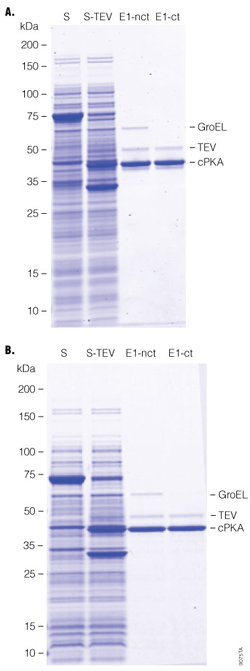 Figure 4. Chaperonin removal.
Figure 4. Chaperonin removal.
Cells expressing the HaloTag®-PKA protein were grown in 2 × 50ml of LB. The cells were collected, resuspended in 5ml of HT buffer, and cell membranes lysed by sonication. In Panel A, a final concentration of 2mM ATP and 10mM MgCl2 were added to the lysates and were incubated at 37°C for 10 minutes. Total lysates were centrifuged at 10,000 × g at 4°C for 30 minutes to collect the soluble supernatant; 5ml of expression cell lysate supernatant was added to 1ml of settled HaloLink™ Resin. After 1 hour of binding at room temperature with end-to-end rotational mixing, HaloLink™ Resin was loaded onto a column (Pierce Cat.# 89897), washed with 20ml (20cv) of HT buffer (Panel A), or 1ml of 2mM ATP and 10mM MgCl2 solution was loaded on to the column and incubated at room temperature for 30 minutes before washing with 20cv of HT buffer (Panel B). One milliliter (1ml) of cleavage solution was loaded onto each column (about 300 units TEV/ml of settled HaloLink™ Resin). TEV Protease cleavage was performed for 1 hour at room temperature; target protein was eluted with HT buffer, and about 2ml of elution was collected. Finally, 50µl of 50% HisLink™ Resin was added to remove TEV Protease, and was incubated for 20 minutes at room temperature with end-to-end rotational mixing. For SDS-PAGE (Invitrogen Tris-Glycine 4–20%): lanes S and S-TEV are equivalent to 10µl of original cell culture, and E1 -nct (without chaperonin treatment) and E1-ct (with chaperonin treatment) are equivalent to 25µl of original cell culture (TEV Protease not removed).
Conclusions
HaloTag®-mediated protein purification is based on covalent immobilization, which enables efficient immobilization even for low abundance fusion proteins. This covalent immobilization also makes extensive washing possible for removing contaminants. HaloTag® technology has been shown to enhance soluble protein expression in E. coli (3) . Thus the HaloTag®-based expression and purification strategy is a convenient and reliable method for the recovery of high-yield, pure, fusion tag-free protein of interest.
Related Protocols
Related Resources
Article References
- Los, G.V. and Wood, K. (2007) The HaloTag: A novel technology for cell imaging and protein analysis. Methods Mol. Biol. 356, 195–208.
- Los, G.V. et al. (2008) HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–82.
- Ohana, R.F. et al. (2009) HaloTag7: A genetically engineered tag that enhances bacterial expression of soluble proteins and improves protein purification. Protein Expr. Purif. 68, 110–20.
- Horwich, A.L. and Fenton, W.A. (2009) Chaperonin-mediated protein folding: Using a central cavity to kinetically assist polypeptide chain folding. Q. Rev. Biophys. 42, 83–116.
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
Zhao, K. Q., Kinney, J., Ohana, R. F. and Urh, M. High Protein Yield and Purity with the HaloTag® Protein Purification System. [Internet] 2010. [cited: year, month, date]. Available from: https://www.promega.com/resources/pubhub/high-protein-yield-and-purity-with-the-halotag-protein-purification-system/
American Medical Association, Manual of Style, 10th edition, 2007
Zhao, K. Q., Kinney, J., Ohana, R. F. and Urh, M. High Protein Yield and Purity with the HaloTag® Protein Purification System. Promega Corporation Web site. https://www.promega.com/resources/pubhub/high-protein-yield-and-purity-with-the-halotag-protein-purification-system/ Updated 2010. Accessed Month Day, Year.
Flexi, GloMax and HaloTag are registered trademarks of Promega Corporation. HaloLink is a trademark of Promega Corporation. His•Tag is a registered trademark of EMD Biosciences. IGEPAL is a registered trademark of Rhone-Poulenc AG Co. Products may be covered by pending or issued patents or may have certain limitations.