Identification of GMOs in Food Using the Maxwell® RSC PureFood GMO and Authentication Kit
Nadine Nassif and Brad Hook
Promega Corporation
Publication Date: 2/17
Abstract
Here we describe the extraction of DNA from a variety of food samples using the Maxwell® PureFood GMO and Authentication Kit and the Maxwell® RSC instrument. The extracted DNA was evaluated for the presence of GM sequences using three qPCR assays: 35S promoter (P35S) from cauliflower mosaic virus (CaMV), ORFIII (third open reading frame) from cauliflower mosaic virus (CaMV) and Universal Plant DNA sequence.
Introduction
Genetically modified organisms (GMOs) are engineered with the goal of producing traits such as herbicide resistance, insect resistance or increased yield. GM crops are primarily grown for use in biofuels, animal feed and clothing; a relatively small percentage of total GMO production is used in food.
In the European Union, regulation of GMOs is based on thresholds for GMO content. The legal threshold for GMO content in food and feed in the EU is 0.9 percent; GMO content below that threshold is excluded from EU labeling requirements (1) . There is no law in the US requiring that foods with GMO ingredients be labeled accordingly; federal legislation has been proposed that would mandate labeling of any food with a GMO ingredient, but this proposed legislation has not advanced (2) .
Quantitative PCR assays are used to determine the amount of a GMO present in a food sample, enabling the verification of GMO thresholds; these quantitative assays are a relatively recent development in the food testing market. PCR-based GMO detection methods can be designed to detect a specific GMO event, or to detect a variety of different GMO events.
Event-specific GMO tests: A GMO “event” refers to a unique genetic alteration used to generate a specific transgenic plant. Event-specific assays are customized to detect specific genetic alterations in individual plants and crops.
Broad-spectrum GMO tests: Many transgenic sequences incorporate the same viral and bacterial genetic elements, to regulate expression of the genetic alterations. Testing for these elements does not reveal a specific GMO event; rather, these broad-spectrum tests can be used to detect a variety of different GMO events. Commonly used transgenic elements include:
- Bt endotoxin sequences: The engineered gene produces an endotoxin protein from the bacterium Bacillus thuringiensis, which is poisonous to certain insect pests. Organic growers commonly use Bt corn as an alternative to spraying insecticides.
- 35S promoter (P35S) from cauliflower mosaic virus (CaMV)
- NOS terminator (TNOS) from Agrobacterium tumefaciens
- 34S promoter (P34S) from figwort mosaic virus (FMV): Monsanto has developed a variety of GM crops with glyphosate herbicide tolerance that utilize the 34S promoter, including MON89788 soy, H7-1 sugar beet and GT73 rapeseed (canola).
To quantitate the GMO level in a particular sample, a combination of broad-spectrum and event-specific qPCR assays is often the most efficient approach (3) .
The Maxwell® RSC PureFood GMO and Authentication Kit used with the Maxwell® RSC Instrument is designed to provide an easy and automated method for efficient purification of DNA used in PCR-based testing for Genetically Modified Organism (GMO) DNA sequences and PCR-based food and ingredient authentication.
The Maxwell® RSC Instrument is supplied with preprogrammed purification methods and is designed for use with predispensed reagent cartridges, maximizing simplicity and convenience. The instrument can purify DNA in approximately 40 minutes from 1 to 16 raw and processed food samples including: corn, soybeans, canola, ground pork, ground beef, pork gelatin, breaded fish, tortillas, corn chips and rice cakes.
In this article, we extracted DNA from a variety of food samples using the Maxwell® PureFood GMO and Authentication Kit and the Maxwell® RSC instrument. The extracted DNA was evaluated for the presence of GM sequences using three qPCR assays:
- 35S promoter (P35S) from cauliflower mosaic virus (CaMV): This amplicon is used to detect the 35S CaMV promoter, which is one of the most commonly used genetic modifications in approved GMOs. This is a broad-spectrum GMO assay.
- ORFIII (third open reading frame) from cauliflower mosaic virus (CaMV): This amplicon is used to detect false positives. The ORFIII sequence would not be present in a GM vector; detection of both the P35S sequence and the ORFIII sequence would indicate a plant that was naturally infected with the CaMV virus.
- Universal Plant DNA sequence: This amplicon is used to confirm the presence of amplifiable plant DNA.
Materials Required
DNA Purification
- Maxwell® RSC Instrument (Cat.# AS4500)
- Maxwell® RSC PureFood GMO and Authentication Kit (Cat.# AS1600)
- Heat block
- Food sample(s)
GMO Detection
- GoTaq® qPCR Master Mix (Cat.# A6001)
- GoTaq® Probe qPCR Master Mix (Cat.# A6101)
- CaMV P35S and CaMV ORFIII control templates
Note: Using these plasmid templates, qPCR standard curves can be generated. This enables calculation of copies per reaction in each sample. Copy numbers for the plasmid templates were calculated using the following equation:
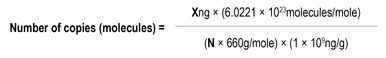
N = length of dsDNA amplicon
660 g/mole = average mass of 1bp dsDNA
Amplification Primers (IDT)
| ORFIII (CaMV) (4) |
|
|
| Fluorescence |
|
|
| Real-Time PCR |
|
|
The Maxwell® RSC PureFood GMO and Authentication Kit Technical Manual #TM473 and the Maxwell® RSC Methods Installation Technical Manual #TM435 are available at www.promega.com/protocols/ or by contacting Technical Services at techserv@promega.com.
Protocol Methods
DNA Purification
- Prepare food sample using a mechanical bead-beating device or grinding method. Samples that are already ground (e.g., flour or ground beef) or that easily break into small pieces do not need additional grinding. However, intact samples need to be finely chopped or ground to allow the reagents to contact all the sample material for better sample disruption.
- Transfer 100mg of each sample into a microcentrifuge tube. When processing a larger volume sample, follow Section 4.C in the Maxwell® RSC PureFood GMO and Authentication Kit Technical Manual #TM473.
- Add 1ml of CTAB buffer to each tube.
- Add 20µl of RNase A Solution and 40µl of Proteinase K (PK) Solution to each tube.
- Vortex tubes until the samples are resuspended.
- Place tubes in a heat block at 65°C for 30 minutes.
- Centrifuge for 10 minutes at maximum speed (16,000 × g).
- Prepare the Maxwell® cartridge deck tray as described in the technical manual.
- Add 100µl of Elution Buffer to the bottom of each Elution Tube and place into the front of the deck tray.
- Add 300µl of Lysis Buffer to well #1 of each cartridge.
- Add 300µl of the processed sample lysate (from Step 7) to well #1 of each cartridge.
- Refer to the Maxwell® RSC Instrument Operating Manual #TM411 for detailed information. To run the RSC PureFood GMO and Authentication protocol, the Maxwell® RSC PureFood GMO and Authentication method must be installed on your instrument. The method is available at: www.promega.com/resources/tools/maxwellrscmethod/. See the Maxwell® RSC Methods Installation Technical Manual #TM435 for instructions.
- Follow the instrument run instructions in the Maxwell® RSC PureFood GMO and Authentication Kit Technical Manual #TM473.
GMO Detection
A. GoTaq® qPCR Master Mix amplification: Universal plant DNA sequence
- Prepare 20X Primer Mix by combining 4µM forward primer and 4µM reverse primer.
- Prepare qPCR Reaction Mix by combining 4µl Nuclease-Free Water, 10µl 2X GoTaq® qPCR Master Mix and 1µl of 20X Primer Mix (volumes per sample).
- Aliquot 15µl of the qPCR Reaction Mix to each reaction well in the amplification plate.
- Add 5µl of purified DNA to each sample well.
- Add 5µl of Nuclease-Free Water to each negative (no template) control well.
- Perform cycling on a real-time instrument following the conditions described in GoTaq® qPCR Master Mix Technical Manual #TM318.
B. GoTaq® Probe qPCR Master Mix amplification: CaMV P35S sequence and CaMV ORFIII sequence
- Prepare 20X Primer Mix by combining 18µM forward primer, 18µM reverse primer and 5µM probe.
- If cycling instrument requires, add reference dye to the GoTaq® Probe qPCR Master Mix (see GoTaq® Probe qPCR Master Mix Technical Manual #TM378 for more information).
- Prepare qPCR Reaction Mix using 4µl Nuclease-Free Water, 10µl 2X GoTaq® qPCR Probe Master Mix and 1µl of 20X Primer Mix (volumes per sample).
- Aliquot 15µl of the qPCR Reaction Mix to each reaction well in the amplification plate.
- Add 5µl of purified DNA to each sample well.
- Add 5µl of Nuclease-Free Water to each negative (no template) control well.
- Perform cycling on a real-time instrument following the conditions described in the GoTaq® Probe qPCR Master Mix Technical Manual #TM378.
Results
The concentration of extracted DNA was determined using the Quantus™ Fluorometer (Cat.# E6150) and the QuantiFluor® ONE dsDNA System (Cat.# E4871). The presence of amplifiable plant DNA was confirmed by amplifying the Universal Plant DNA sequence using dye-based qPCR (GoTaq® qPCR Master Mix). Concentration (ng/µl) and Cq values are shown in Table 1.
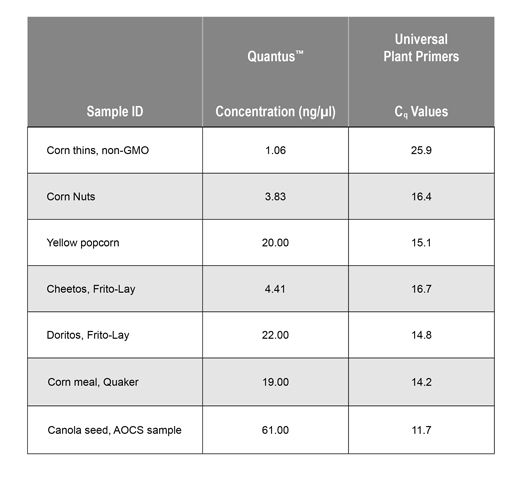
The presence of GMO DNA was determined by amplifying the CaMV P35S sequence using probe-based qPCR. This broad-spectrum assay will detect GMOs that were generated using the cauliflower mosaic virus promoter element, but will not detect all possible GMO events.
Samples were also screened for false positive reactions by amplifying the CaMV ORFIII sequence using probe-based qPCR. Detection of both the P35S sequence and the ORFIII sequence indicates a plant that was naturally infected with the CaMV virus.
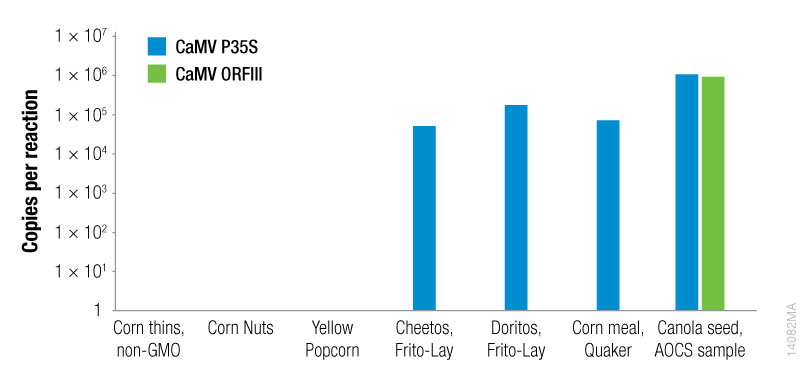
To quantitate the copies per reaction for the CaMV P35S and CaMV ORFIII sequences, standard curves were generated for each sequence using the plasmid templates (Figures 1 and 2).
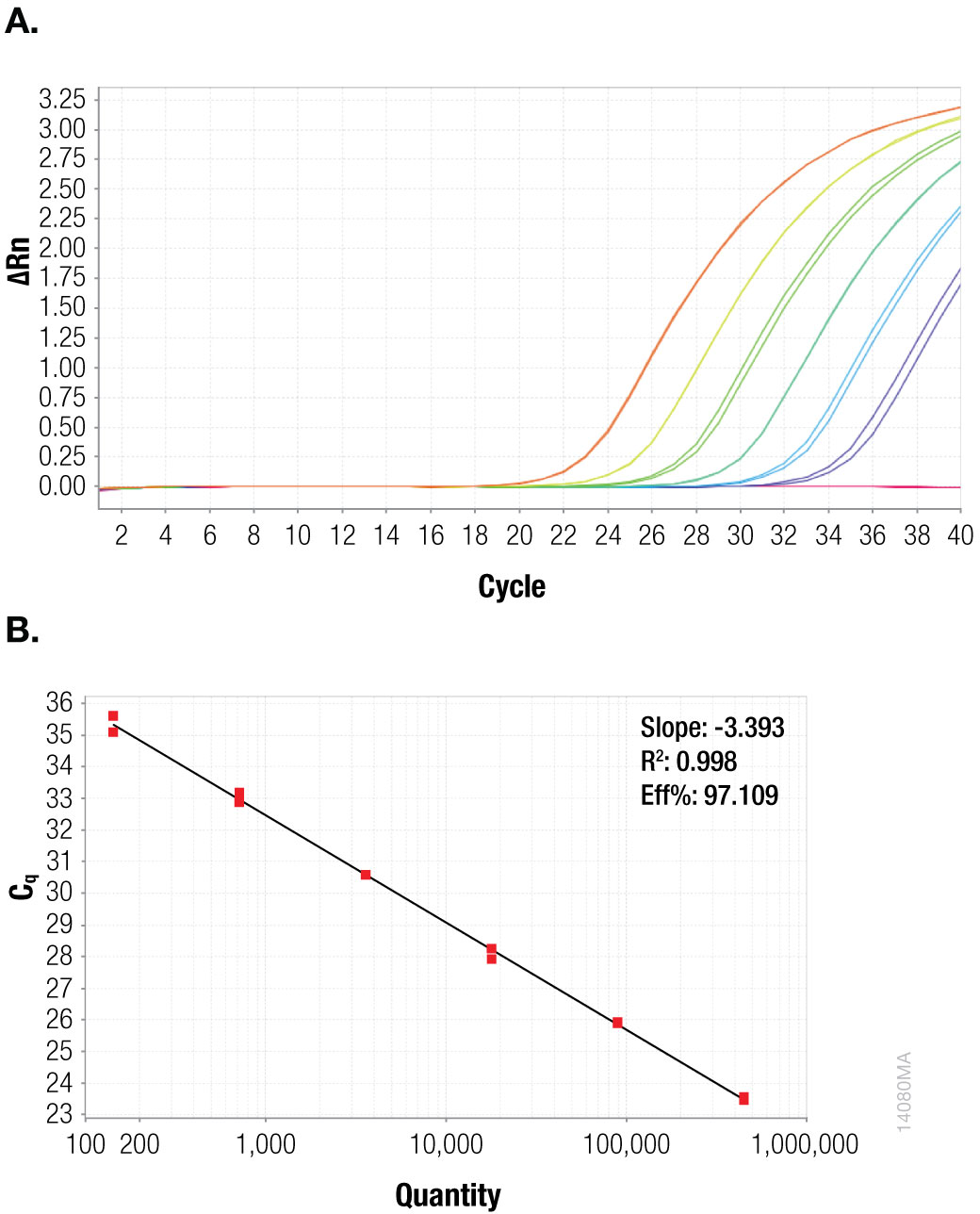
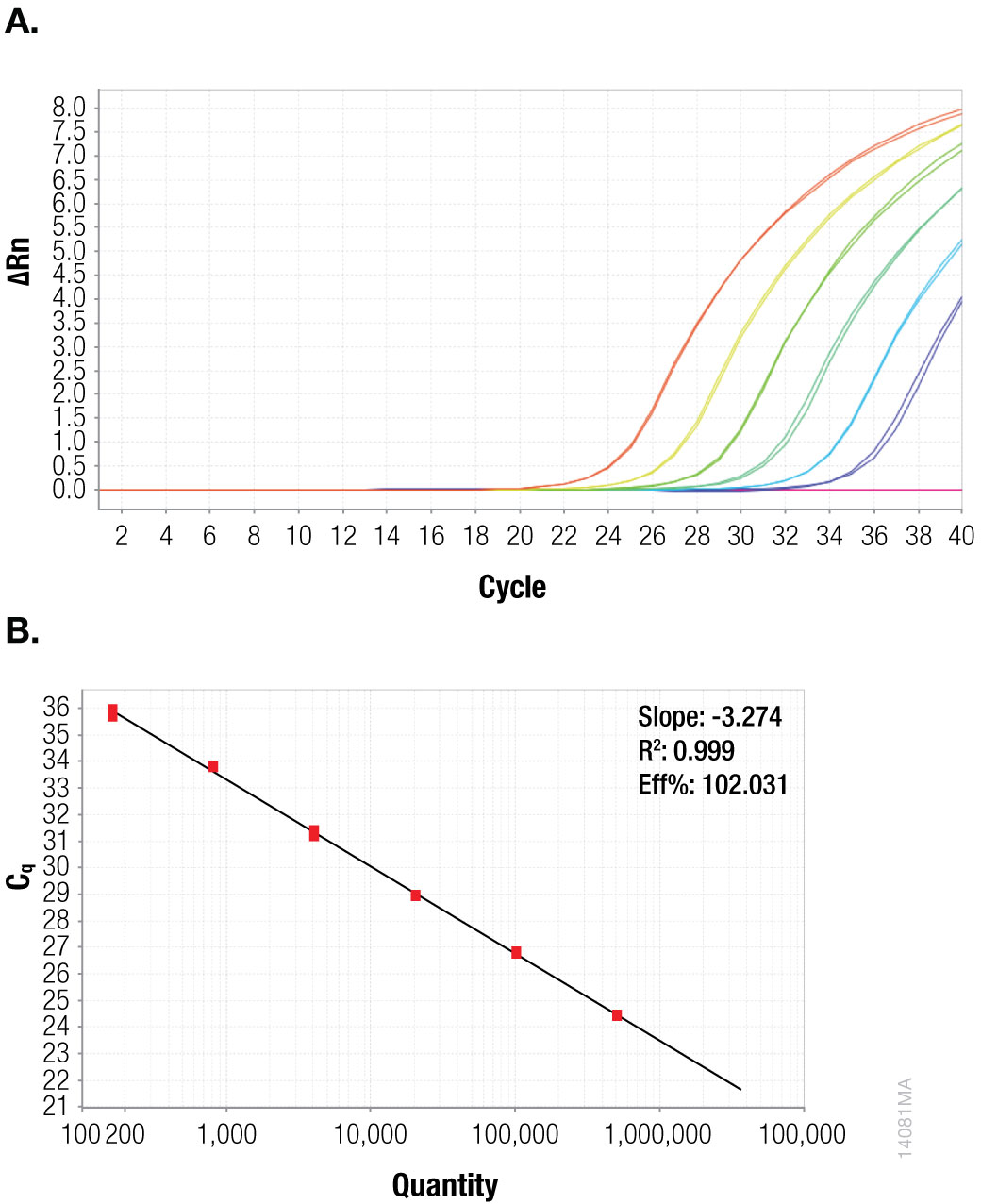
Figure 2. CaMV ORFIII Fivefold serial dilution of plasmid template, starting at 507,500 copies per reaction. Panel A. Amplification plot. Panel B. Standard curve.
Based on the standard curves for the CaMV P35S and CaMV ORFIII sequences, the copies per reaction for each sequence were determined for all samples (Figure 3). The promoter sequence (CaMV P35S) was detected in 4 of the 7 samples. Among the samples tested, one sample tested positive for both the P35S sequence and the ORFIII sequence, indicating that the plant had been naturally infected with CaMV. The sample (canola seed, AOCS) was a certified reference sample, known to be generated using the figwort mosaic virus (FMV) promoter element.
Summary
GMO labeling laws vary from country to country around the world. While there is no law in the United States that requires labeling of foods with GMO ingredients, the European Union (as well as other countries) have implemented mandatory labeling of most GMO foods.
The Maxwell® PureFood GMO and Authentication Kit proved to be an efficient method for isolating DNA from the food samples tested in these experiments. The DNA eluates were free from significant amounts of inhibitors (data not shown) and robust amplification was observed when performing qPCR to detect the presence of Universal Plant DNA.
These results highlight an important aspect of GMO testing: a single broad-spectrum assay cannot detect all possible GMOs. In these experiments, our assay can only confirm the presence or absence of the cauliflower mosaic virus promoter element; we cannot conclusively determine that a sample is “non-GMO” without further testing to assess for the presence of other GM elements. Additionally, detecting the presence of a GM element is insufficient to qualify the sample as requiring GMO labeling; the percentage of GM content would need to be assessed to make this determination.
References
- (2015, June 9) Restrictions on Genetically Modified Organisms: European Union.
- (2015, June 9) Restrictions on Genetically Modified Organisms: United States.
- Luque-Perez, E. et al. (2012) Testing the robustness of validated methods for quantitative detection of GMOs across qPCR instruments. Food Anal Method. 6, 343–60.
- Chaouachi, M. et al. (2008) An accurate real-time PCR test for the detection and quantification of cauliflower mosaic virus (CaMV): Applicable in GMO screening. Eur Food Res Technol. 227, 789–98.